
Write two characteristics observed in photoelectric effect which support the photon picture of electromagnetic radiation. Draw a graph between the frequency of incident radiation (\[\nu \]) and the maximum kinetic energy of the electrons emitted from the surface of a photosensitive material. State clearly how this graph can be used to determine (i) Planck’s constant and (ii) work function of the material.
Answer
569.1k+ views
Hint:According to the photoelectric effect, when the photon having the energy greater than the work function of the material strikes the material surface, it knocks out the inner shell electron from the atom. Recall the characteristics features observed in photoelectric effect which support the photon picture of electromagnetic radiation. Draw the graph between kinetic energy and frequency of the electron. The energy of the photon is equal to Planck’s constant times the frequency of the photon.
Complete answer:
To answer this question, let’s recall the photoelectric effect. When the photon having the energy greater than the work function of the material strikes the material surface, it knocks out the inner shell electron from the atom. This phenomenon is known as the photoelectric effect.
Let’s discuss the characteristics observed in photoelectric effect.
The maximum kinetic energy of the photoelectron emitted from the material is independent of the incident intensity of the photon and solely depends on the frequency of the incident photon.For every metal, the photo-electric emission will not take place if the frequency of the incident photon is less than the threshold frequency. If the frequency of the photon is less than a certain minimum frequency known as threshold frequency, the photo-electric emission will not happen.
Let’s draw a graph between the frequency of incident radiation (\[\nu \]) and the maximum kinetic energy of the electrons emitted from the surface of a photosensitive material as shown in the figure below.
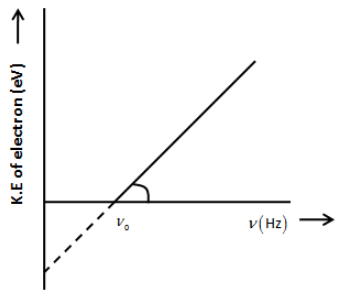
(i) We know that the energy of the photon is expressed as,
\[K.E = h\nu \]
\[ \Rightarrow h = \dfrac{{K.E}}{\nu }\]
Here, h is Planck's constant. Therefore, the Planck’s constant is the ratio of the kinetic energy and frequency of the photon.
From the above graph, the slope of the curve is,
\[m = \dfrac{{\Delta K.E}}{{\Delta \nu }}\]
From the above two equations, we can clearly say that the slope of the curve is K.E vs. frequency graph is the Planck’s constant. Therefore, the Planck’s constant is,
\[h = \dfrac{{\Delta K.E}}{{\Delta \nu }}\]
(ii) We know that the work function is the minimum energy required to eject the electron from the metal. We know that the energy of the photon is, \[K.E = h\nu \]. We have seen that the photon can emit the electron when the frequency of the photon reaches the threshold frequency\[{\nu _0}\]. Thus, the minimum energy of the photon that is the work function of the material is expressed as,
\[\psi = h{\nu _0}\]
From the above graph, we can easily calculate the work function of the metal.
Note:The graph of kinetic energy and frequency shows that the curve does not start from the origin because the photo-emission does not happen at 0 frequency of the photon. Do not get confused between Compton Effect and photoelectric effect. In the Compton Effect, the incident photon knocks out electrons from the outer shell as well as it emits photons with less energy than the incident photon.
Complete answer:
To answer this question, let’s recall the photoelectric effect. When the photon having the energy greater than the work function of the material strikes the material surface, it knocks out the inner shell electron from the atom. This phenomenon is known as the photoelectric effect.
Let’s discuss the characteristics observed in photoelectric effect.
The maximum kinetic energy of the photoelectron emitted from the material is independent of the incident intensity of the photon and solely depends on the frequency of the incident photon.For every metal, the photo-electric emission will not take place if the frequency of the incident photon is less than the threshold frequency. If the frequency of the photon is less than a certain minimum frequency known as threshold frequency, the photo-electric emission will not happen.
Let’s draw a graph between the frequency of incident radiation (\[\nu \]) and the maximum kinetic energy of the electrons emitted from the surface of a photosensitive material as shown in the figure below.

(i) We know that the energy of the photon is expressed as,
\[K.E = h\nu \]
\[ \Rightarrow h = \dfrac{{K.E}}{\nu }\]
Here, h is Planck's constant. Therefore, the Planck’s constant is the ratio of the kinetic energy and frequency of the photon.
From the above graph, the slope of the curve is,
\[m = \dfrac{{\Delta K.E}}{{\Delta \nu }}\]
From the above two equations, we can clearly say that the slope of the curve is K.E vs. frequency graph is the Planck’s constant. Therefore, the Planck’s constant is,
\[h = \dfrac{{\Delta K.E}}{{\Delta \nu }}\]
(ii) We know that the work function is the minimum energy required to eject the electron from the metal. We know that the energy of the photon is, \[K.E = h\nu \]. We have seen that the photon can emit the electron when the frequency of the photon reaches the threshold frequency\[{\nu _0}\]. Thus, the minimum energy of the photon that is the work function of the material is expressed as,
\[\psi = h{\nu _0}\]
From the above graph, we can easily calculate the work function of the metal.
Note:The graph of kinetic energy and frequency shows that the curve does not start from the origin because the photo-emission does not happen at 0 frequency of the photon. Do not get confused between Compton Effect and photoelectric effect. In the Compton Effect, the incident photon knocks out electrons from the outer shell as well as it emits photons with less energy than the incident photon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




