
Write trans-esterification reaction.
Answer
594.9k+ views
Hint: The final product of transesterification reaction is an ester and an alcohol. The reactants are also an ester and an alcohol but products and reactants are not the same.
Complete step by step solution:
- It is known to you that when an alcohol (generally primary alcohol) is treated with carboxylic acid in the presence of sulphuric acid, a sweet smelling compound is formed which is called ester. The reaction is known as the esterification reaction.
The general formula of ester is RCOOR and the general reaction of esterification is as follows:
\[RCOOH + R'OH \to RCOOR' + {H_2}O\]
- Transesterification is the process of exchanging the organic group R of an ester with the organic group R’ of an alcohol. These reactions are catalyzed in the presence of an acid or base catalyst. The general reaction of transesterification is as follows:
\[RCOOR' + R'' - OH\xrightarrow{{{H^ + }o{r^ - }OH}}RCOOR'' + R'OH\]
- In transesterification, strong acids catalyze the reaction by donating a proton to the carbonyl group , thus making it a potent electrophile, whereas base catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophile.
- Example of transesterification reaction is as below.
\[\mathop {C{H_3}COOC{H_3}}\limits_{{\text{Methyl acetate}}} + \mathop {C{H_3}C{H_2}OH}\limits_{{\text{Ethanol}}} \xrightarrow{{{H^ + }o{r^ - }OH}}\mathop {C{H_3}COOC{H_2}C{H_3}}\limits_{{\text{Ethyl acetate}}} + \mathop {C{H_3}OH}\limits_{Methanol} \]
- Mechanism of this reaction:
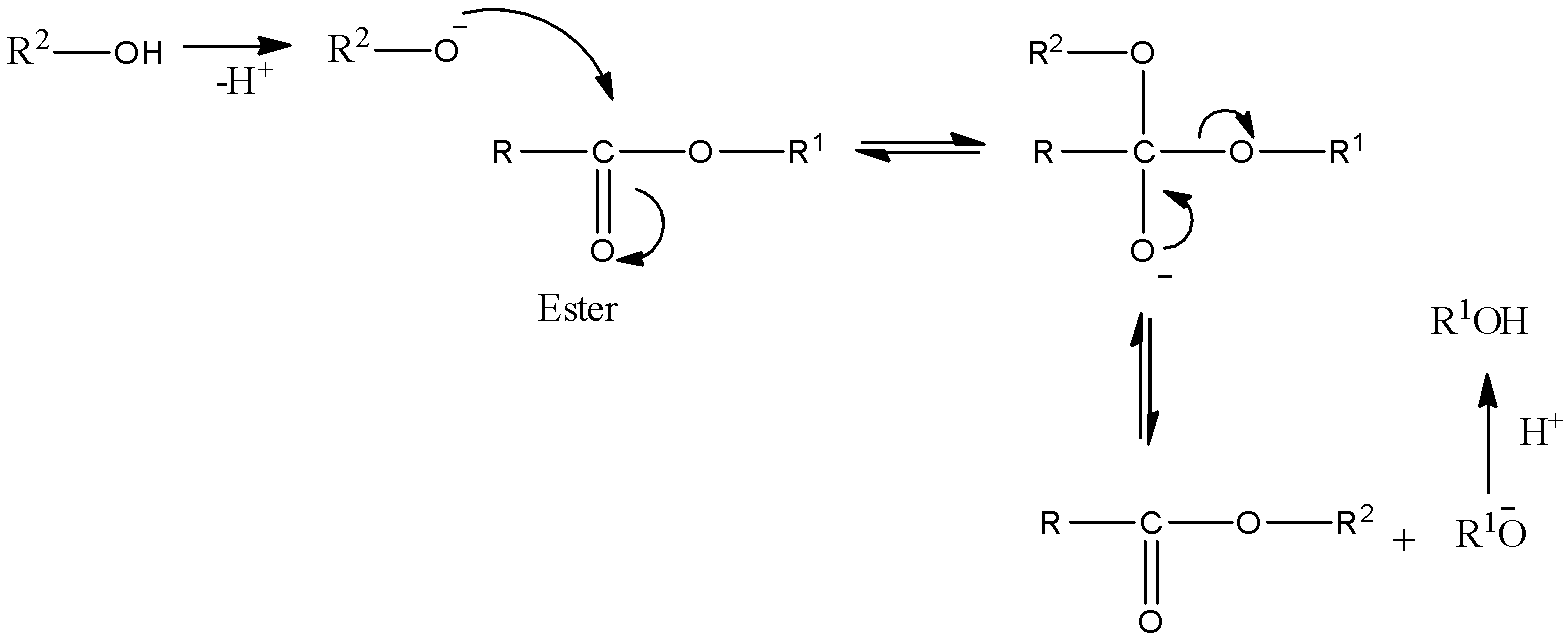
- Here, we can see that firstly, the alkoxy group of alcohol attacks the carbonyl carbon of the ester. Then simultaneously the other alkoxy group gets removed and a new ester gets formed. Alcohol formed is also different from the reactant.
- Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to derive equilibrium.
- Here, we can see that firstly, the alkoxy group of alcohol attacks the carbonyl carbon of the ester. Then simultaneously the other alkoxy group gets removed and a new ester gets formed. Alcohol formed is also different from the reactant.
- Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to derive equilibrium.
Note: Remember that in transesterification reaction, the alkyl group that is directly attached to the carbonyl carbon does not get replaced by the alkyl group of alcohol because it is not reactive in terms of reaction with alcohol.
Complete step by step solution:
- It is known to you that when an alcohol (generally primary alcohol) is treated with carboxylic acid in the presence of sulphuric acid, a sweet smelling compound is formed which is called ester. The reaction is known as the esterification reaction.
The general formula of ester is RCOOR and the general reaction of esterification is as follows:
\[RCOOH + R'OH \to RCOOR' + {H_2}O\]
- Transesterification is the process of exchanging the organic group R of an ester with the organic group R’ of an alcohol. These reactions are catalyzed in the presence of an acid or base catalyst. The general reaction of transesterification is as follows:
\[RCOOR' + R'' - OH\xrightarrow{{{H^ + }o{r^ - }OH}}RCOOR'' + R'OH\]
- In transesterification, strong acids catalyze the reaction by donating a proton to the carbonyl group , thus making it a potent electrophile, whereas base catalyse the reaction by removing a proton from the alcohol, thus making it more nucleophile.
- Example of transesterification reaction is as below.
\[\mathop {C{H_3}COOC{H_3}}\limits_{{\text{Methyl acetate}}} + \mathop {C{H_3}C{H_2}OH}\limits_{{\text{Ethanol}}} \xrightarrow{{{H^ + }o{r^ - }OH}}\mathop {C{H_3}COOC{H_2}C{H_3}}\limits_{{\text{Ethyl acetate}}} + \mathop {C{H_3}OH}\limits_{Methanol} \]
- Mechanism of this reaction:

- Here, we can see that firstly, the alkoxy group of alcohol attacks the carbonyl carbon of the ester. Then simultaneously the other alkoxy group gets removed and a new ester gets formed. Alcohol formed is also different from the reactant.
- Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to derive equilibrium.
- Here, we can see that firstly, the alkoxy group of alcohol attacks the carbonyl carbon of the ester. Then simultaneously the other alkoxy group gets removed and a new ester gets formed. Alcohol formed is also different from the reactant.
- Esters with larger alkoxy groups can be made from methyl or ethyl esters in high purity by heating the mixture of ester, acid/base, and large alcohol and evaporating the small alcohol to derive equilibrium.
Note: Remember that in transesterification reaction, the alkyl group that is directly attached to the carbonyl carbon does not get replaced by the alkyl group of alcohol because it is not reactive in terms of reaction with alcohol.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




