
Write the symbol / formula of the following
A.A neutral oxide
B.Period 2 element showing diagonal relationship to aluminium from period 3
C.Halogen having maximum negative electron gain enthalpy in the group
Answer
571.5k+ views
Hint: Oxides are chemical compounds with one or more oxygen atoms combined with other elements.Oxides are also defined as the binary compounds of oxygen with other elements. Based on acid base characteristics of oxides, they are classified as acidic, basic, amphoteric, or neutral.
Complete step by step solution:
A.It's already based on their acid base characteristic oxides are classified into acidic, basic, amphoteric or neutral.
An oxide that combine with water and give an acid termed as acidic oxide
An oxide which gives base when react with water called basic oxide
Neutral oxides are those which show neither acidic nor basic properties whey they react with water.
Examples for such neutral oxide are carbon monoxide ($CO$), nitrous oxide ($N_2O$)
So here the neutral oxides are $CO$, $N_2O$
Neutral substance is a substance which shows no acid or base properties, and has an equal number of hydrogen and hydroxyl ions and it does not change the colour of litmus paper. Neutral oxides $CO$ and $N_2O$ are slightly soluble in water.
B.When an element which shows similarities to another element in the next higher group and in the next period, these elements are said to exhibit diagonal relationship. In other words we can say a diagonal relationship is said to exist between certain pairs which are diagonally adjacent elements in the second and their periods of the periodic table.
Some elements of the second period show similarities with elements of the third period called a diagonal relationship.
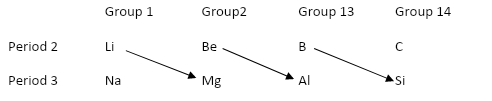
From above it is clear that the element of period 2 which shows diagonal relationship to Al in period 3 is Beryllium(Be).
Cause of diagonal relationship is because when moving along the period from left to right the electronegativity is found to be increased, also moving down the group electropositivity of elements increases. The two effects will tend to cancel each other as moving diagonally from top left to bottom right.so that the elements diagonally related in this way tend to have similar properties.
So the element shows diagonal relationship with Al is Be.
Since Al and Be show diagonal relationship they both have some similar properties. some are given below
Both elements dissolve in strong alkali to liberate hydrogen.
Both have a strong tendency to form covalent compounds.
Both form non volatile, hard oxide and have very high melting points.
Both have the same electronegativity and polarising power.
Both BeCl2 and AlCl3 have chlorine bridged structures in vapor state.
C.Electrons gain enthalpy ( \[{\vartriangle _{eg}}H\]) of an element is defined as energy released when a neutral isolated gaseous atom accepts an extra electron to form gaseous negative ions. Greater the amount of energy released in the above process, higher is the electron gain enthalpy of element is a measure of the strength with which an extra electron is bound to it.
The energy released when a neutral isolated gaseous atom accepts an extra electron. Here energy is released when an electron is added to the atom, therefore the electron gain enthalpy is negative.
\[{X_{\left( g \right)}} + \,{e^ - }\, \to {X^ - }\] ${\vartriangle _{eg}}H$ is the electron gain enthalpy.
The electron gain enthalpy for halogens is highly negative because they can acquire the nearest stable noble gas configuration by accepting an extra electron.
The electron gain enthalpy of halogens is highly negative, because they can get the nearest stable noble gas configuration by accepting an extra electron. The variation of electron gain enthalpy with in halogen group is shown below
The correct order of electron gain enthalpy of halogen group elements is Cl > F > Br > I
As we move down the group, both atomic size and the nuclear charge increases, but the effect of the increase in atomic size is more pronounced than the nuclear charge. As atomic size increases the attraction nucleus to the coming electron decreases hence electron gain enthalpy is less negative down the group.
The electron gain enthalpy of fluorine is less negative than that of chlorine, it is due to the small size of fluorine. As a result of the small size of fluorine electron -electron repulsion in the 2p subshell is large and hence the incoming electron is not accepted easily as in case of chlorine.
So maximum negative electron gain enthalpy is for Cl.
Note: Electron gain enthalpy is measured in electron volts per atom. The process of adding an electron to an atom can be exothermic or endothermic. The factors affecting electron gain enthalpy are atomic size, nuclear charge and electronic configuration
Complete step by step solution:
A.It's already based on their acid base characteristic oxides are classified into acidic, basic, amphoteric or neutral.
An oxide that combine with water and give an acid termed as acidic oxide
An oxide which gives base when react with water called basic oxide
Neutral oxides are those which show neither acidic nor basic properties whey they react with water.
Examples for such neutral oxide are carbon monoxide ($CO$), nitrous oxide ($N_2O$)
So here the neutral oxides are $CO$, $N_2O$
Neutral substance is a substance which shows no acid or base properties, and has an equal number of hydrogen and hydroxyl ions and it does not change the colour of litmus paper. Neutral oxides $CO$ and $N_2O$ are slightly soluble in water.
B.When an element which shows similarities to another element in the next higher group and in the next period, these elements are said to exhibit diagonal relationship. In other words we can say a diagonal relationship is said to exist between certain pairs which are diagonally adjacent elements in the second and their periods of the periodic table.
Some elements of the second period show similarities with elements of the third period called a diagonal relationship.

From above it is clear that the element of period 2 which shows diagonal relationship to Al in period 3 is Beryllium(Be).
Cause of diagonal relationship is because when moving along the period from left to right the electronegativity is found to be increased, also moving down the group electropositivity of elements increases. The two effects will tend to cancel each other as moving diagonally from top left to bottom right.so that the elements diagonally related in this way tend to have similar properties.
So the element shows diagonal relationship with Al is Be.
Since Al and Be show diagonal relationship they both have some similar properties. some are given below
Both elements dissolve in strong alkali to liberate hydrogen.
Both have a strong tendency to form covalent compounds.
Both form non volatile, hard oxide and have very high melting points.
Both have the same electronegativity and polarising power.
Both BeCl2 and AlCl3 have chlorine bridged structures in vapor state.
C.Electrons gain enthalpy ( \[{\vartriangle _{eg}}H\]) of an element is defined as energy released when a neutral isolated gaseous atom accepts an extra electron to form gaseous negative ions. Greater the amount of energy released in the above process, higher is the electron gain enthalpy of element is a measure of the strength with which an extra electron is bound to it.
The energy released when a neutral isolated gaseous atom accepts an extra electron. Here energy is released when an electron is added to the atom, therefore the electron gain enthalpy is negative.
\[{X_{\left( g \right)}} + \,{e^ - }\, \to {X^ - }\] ${\vartriangle _{eg}}H$ is the electron gain enthalpy.
The electron gain enthalpy for halogens is highly negative because they can acquire the nearest stable noble gas configuration by accepting an extra electron.
The electron gain enthalpy of halogens is highly negative, because they can get the nearest stable noble gas configuration by accepting an extra electron. The variation of electron gain enthalpy with in halogen group is shown below
The correct order of electron gain enthalpy of halogen group elements is Cl > F > Br > I
As we move down the group, both atomic size and the nuclear charge increases, but the effect of the increase in atomic size is more pronounced than the nuclear charge. As atomic size increases the attraction nucleus to the coming electron decreases hence electron gain enthalpy is less negative down the group.
The electron gain enthalpy of fluorine is less negative than that of chlorine, it is due to the small size of fluorine. As a result of the small size of fluorine electron -electron repulsion in the 2p subshell is large and hence the incoming electron is not accepted easily as in case of chlorine.
So maximum negative electron gain enthalpy is for Cl.
Note: Electron gain enthalpy is measured in electron volts per atom. The process of adding an electron to an atom can be exothermic or endothermic. The factors affecting electron gain enthalpy are atomic size, nuclear charge and electronic configuration
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




