
Write the structure of propanol.
Answer
590.7k+ views
Hint: Propanol is primary alcohol with general structure RCOH where R is alkyl group and –OH is the functional group. It is also known as ethyl carbinol.
Complete step by step answer:
Propanol has chemical formula \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\] or ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}$ .
Propanol is also known as ${\text{propan - 1 - ol}}$ or n-propanol. Its structure is given below-
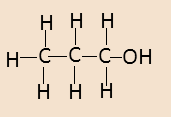
It has a simple straight chain structure. Its properties are as follows-
1.It is a clear colorless liquid.
2.It has a musty odor similar to ethanol or rubbing alcohol.
3.It has a ripe, fruity flavor.
4.It is highly flammable.
5.It is soluble in acetone and benzene and miscible with other organic solvents.
5.It is also soluble in water.
6.It is found in apple, cognac, apricot, banana, papaya, pineapple and cherry.
It is prepared by hydrogenation of propionaldehyde. The reaction is as follows-
$ \Rightarrow C{H_3}C{H_2}CHO + {H_2} \to C{H_3}C{H_2}C{H_2}OH$
In this reaction the two hydrogen molecules get attached to the carbon of the aldehyde functional group and propanol is obtained. It is easily ignited by heat and its vapors form explosive mixtures with air. Its vapours are heavier than air so they can easily spread along the ground and collect in low confined areas.
Note:
The uses of propanol are-
1.As a solvent for natural gums, polishes, pharmaceuticals.
2.In cosmetics, dental lotions and lacquers.
3.As a specialty solvent in printing inks for printing on polyolefin and polyamide film.
4.In manufacture of n-propyl acetate and glycol ethers.
5.As a chemical intermediate in reactions.
6.As a flavoring agent and fragrance in foods.
7.As a co-solvent in pesticide formulation.
Complete step by step answer:
Propanol has chemical formula \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\] or ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}{\text{O}}$ .
Propanol is also known as ${\text{propan - 1 - ol}}$ or n-propanol. Its structure is given below-

It has a simple straight chain structure. Its properties are as follows-
1.It is a clear colorless liquid.
2.It has a musty odor similar to ethanol or rubbing alcohol.
3.It has a ripe, fruity flavor.
4.It is highly flammable.
5.It is soluble in acetone and benzene and miscible with other organic solvents.
5.It is also soluble in water.
6.It is found in apple, cognac, apricot, banana, papaya, pineapple and cherry.
It is prepared by hydrogenation of propionaldehyde. The reaction is as follows-
$ \Rightarrow C{H_3}C{H_2}CHO + {H_2} \to C{H_3}C{H_2}C{H_2}OH$
In this reaction the two hydrogen molecules get attached to the carbon of the aldehyde functional group and propanol is obtained. It is easily ignited by heat and its vapors form explosive mixtures with air. Its vapours are heavier than air so they can easily spread along the ground and collect in low confined areas.
Note:
The uses of propanol are-
1.As a solvent for natural gums, polishes, pharmaceuticals.
2.In cosmetics, dental lotions and lacquers.
3.As a specialty solvent in printing inks for printing on polyolefin and polyamide film.
4.In manufacture of n-propyl acetate and glycol ethers.
5.As a chemical intermediate in reactions.
6.As a flavoring agent and fragrance in foods.
7.As a co-solvent in pesticide formulation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




