
Write the structural formula of propyne.
Answer
560.4k+ views
Hint:We know that alkynes are organic molecules which are made of the functional group carbon-carbon triple bonds and are written in the empirical formula of ${C_n}{H_{2n - 2}}$, where ‘n’ equals any integer greater than one and propyne which is also known as methylacetylene is an alkyne with three carbon atoms. Its formula is ${C_3}{H_4}$.
Complete answer:
Propyne is an alkyne having three carbon atoms and a carbon-carbon triple bond. The formula of propyne is ${C_3}{H_4}$ and its structural formula is given below.
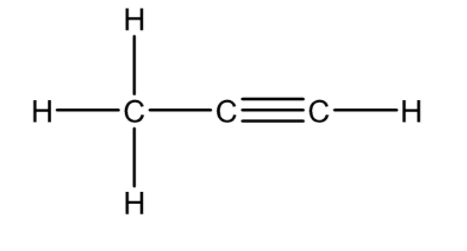
1-propyne, or prop-1-yne or simply propyne appears as a colourless liquefied gas with a sweet odour. Its melting point is ${104^0}C$, and boiling point is ${23.1^0}C$. It is insoluble in water but soluble in ethanol, chloroform and benzene. It is moderately toxic by inhalation and is used as a specialty fuel. It is denser than air. Vapours may ignite at distant sources of ignition and flash back.
Note:
Propyne is an alkyne, a terminal acetylenic compound and a gas molecular entity. Its molecular weight is $40\,g/mol$. Propyne is a convenient three-carbon building block for organic synthesis. The deprotonation with n-butyllithium gives us propynyllithium. This nucleophilic reagent when added to carbonyl groups, produces alcohols and esters. Whereas the purified propyne is expensive, MAPP gas could be used to cheaply generate large amounts of the reagent. Propyne can also be synthesized on the laboratory scale by reducing 1-propanol, allyl alcohol or acetone, vapours over magnesium (Mg). Propyne, along with 2-butyne, is also used to synthesize alkylated hydroquinones in the total synthesis of vitamin E.
Complete answer:
Propyne is an alkyne having three carbon atoms and a carbon-carbon triple bond. The formula of propyne is ${C_3}{H_4}$ and its structural formula is given below.

1-propyne, or prop-1-yne or simply propyne appears as a colourless liquefied gas with a sweet odour. Its melting point is ${104^0}C$, and boiling point is ${23.1^0}C$. It is insoluble in water but soluble in ethanol, chloroform and benzene. It is moderately toxic by inhalation and is used as a specialty fuel. It is denser than air. Vapours may ignite at distant sources of ignition and flash back.
Note:
Propyne is an alkyne, a terminal acetylenic compound and a gas molecular entity. Its molecular weight is $40\,g/mol$. Propyne is a convenient three-carbon building block for organic synthesis. The deprotonation with n-butyllithium gives us propynyllithium. This nucleophilic reagent when added to carbonyl groups, produces alcohols and esters. Whereas the purified propyne is expensive, MAPP gas could be used to cheaply generate large amounts of the reagent. Propyne can also be synthesized on the laboratory scale by reducing 1-propanol, allyl alcohol or acetone, vapours over magnesium (Mg). Propyne, along with 2-butyne, is also used to synthesize alkylated hydroquinones in the total synthesis of vitamin E.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




