
Write the product of the given reaction:
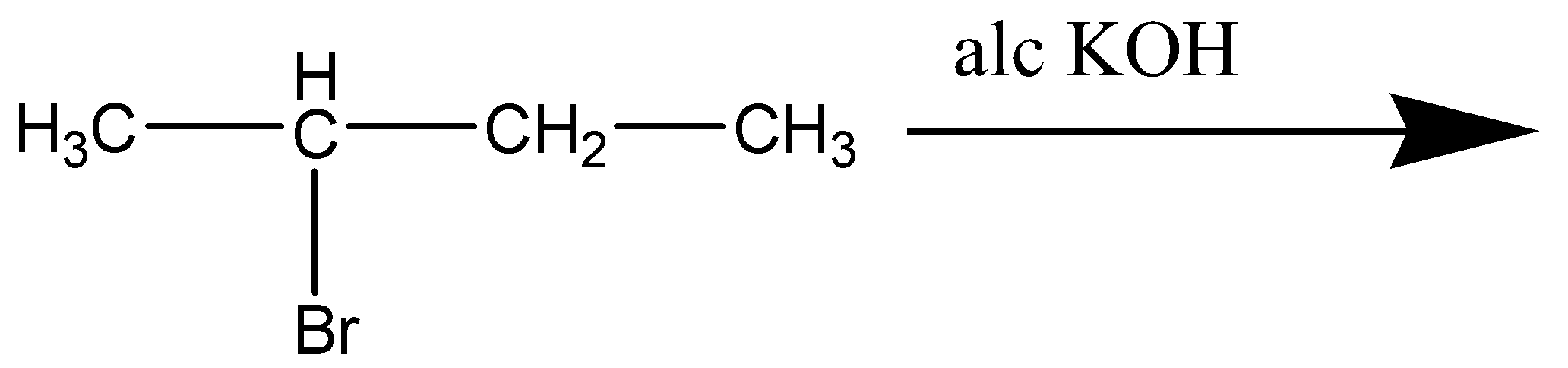

Answer
576k+ views
Hint: Haloalkanes give alkene when they are heated with alcoholic potassium hydroxide (KOH), it is an example of Dehydrohalogenation reaction. The formation of more substituted alkene will be favoured here. So, when the 2- bromobutane is heated with alcoholic KOH we will get a major yield of But-2-ene and minor yield of But-1-ene.
Complete step by step answer:
According to Saytzeff rule, when an alkyl halide is heated or undergoes elimination the major product formed is Alkene.
Formation of bu-2-tene as the major product which can be explained by Saytzeff’s rule which states that if an alkyl halide undergoes elimination in two different ways, then the more highly substituted alkene i.e. having lesser number of hydrogens on the doubly bonded carbon atoms, is the major product of dehydrohalogenation reaction.
When 1-bromobutane reacts in the presence of alcoholic KOH, then it undergoes dehydrohalogenation to form 1-Butene as a product.
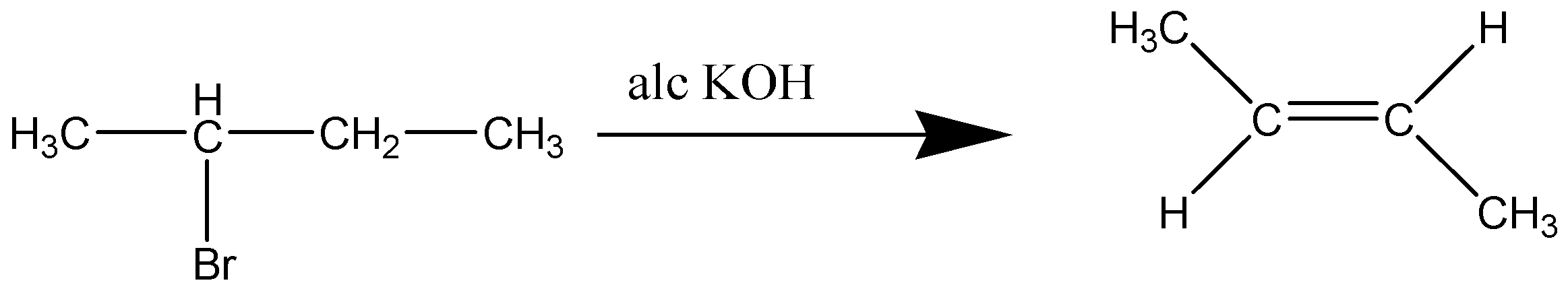
When 2-Bromobutane is heated with alcoholic KOH, So, but-2-ene is a more substituted group, so it will be formed as a major yield or product and a minor yield of But-1-ene .
Since there are three hydrogens and two hydrogens on beta-carbons in 2-bromobutane, then a ratio of 1-butene and 2-butene in the products.
Thus, But-2-ene is the major product formed
Note: By using the strongly basic hydroxide nucleophile, this reaction is directed toward elimination. In this case there are beta-hydrogens available to the elimination reaction. The main factor contributing to Saytzeff’s Rule behaviour is the stability of the alkene. We know that carbon-carbon double bonds are stabilized (thermodynamically) by alkyl substituents, and that this stabilization could be evaluated by appropriate heat of hydrogenation measurements.
Complete step by step answer:
According to Saytzeff rule, when an alkyl halide is heated or undergoes elimination the major product formed is Alkene.
Formation of bu-2-tene as the major product which can be explained by Saytzeff’s rule which states that if an alkyl halide undergoes elimination in two different ways, then the more highly substituted alkene i.e. having lesser number of hydrogens on the doubly bonded carbon atoms, is the major product of dehydrohalogenation reaction.
When 1-bromobutane reacts in the presence of alcoholic KOH, then it undergoes dehydrohalogenation to form 1-Butene as a product.

When 2-Bromobutane is heated with alcoholic KOH, So, but-2-ene is a more substituted group, so it will be formed as a major yield or product and a minor yield of But-1-ene .
Since there are three hydrogens and two hydrogens on beta-carbons in 2-bromobutane, then a ratio of 1-butene and 2-butene in the products.
Thus, But-2-ene is the major product formed
Note: By using the strongly basic hydroxide nucleophile, this reaction is directed toward elimination. In this case there are beta-hydrogens available to the elimination reaction. The main factor contributing to Saytzeff’s Rule behaviour is the stability of the alkene. We know that carbon-carbon double bonds are stabilized (thermodynamically) by alkyl substituents, and that this stabilization could be evaluated by appropriate heat of hydrogenation measurements.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




