
Write the number of covalent bonds in the molecule of ethane.
Answer
598.8k+ views
Hint: Covalent bonds are formed through sharing of electrons between a pair of atoms. . In ethane there are 2 carbon atoms and 3 hydrogen atoms are attached to each of the carbon atoms. All the bonds present in the structure of ethane are covalent as they are sharing their electrons with each other in order to fulfil their octet and gain stability
Complete answer:
Ethane is an alkane i.e. the carbon atoms are singly bonded to each other. The formula of ethane is ${{C}_{6}}{{H}_{6}}$.
We know that covalent bonds are formed by sharing of electrons between two atoms. Atoms which do not have their valence shells fulfilled forms covalent bonding with a similar atom to complete its octet and hence gain stability.
Generally, there are three types of covalent bonding that we know about. They are-
Single bond- When there is a sharing of two electrons between a pair of atoms and gives rise to a sigma bond between the atoms, the sigma bond formed is the single bond.
Double bond- When four electrons are shared by the two atoms, it gives rise to a sigma bond and a pi-bond which is known as the double bond.
Triple bond- When six electrons are shared by the two atoms, there exist one sigma and two pi-bonds thus forming a triple bond.
In ethane, we have a carbon-carbon bold and six carbon-hydrogen bonds.
We know that the atomic number of carbon is 6, which means it has 4 electrons in its valence shell. It needs to share 4 electrons to gain stability by completing its octet and atomic number of hydrogen is 1 and it will need 1 electron. The structure of ethane is-
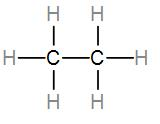
Here, three hydrogen atoms are attached to one carbon atom and the two carbon atoms are bonded to each other too to fulfil their octets. Therefore, all of the bonds present here are covalent and as we can see, there are 7 bonds in total.
Therefore, in ethane there are 7 covalent bonds.
Note:
In order to form a covalent bond, the atoms need to be in a specific arrangement which will allow the overlapping between the orbitals. It is difficult to break a sigma bond because sigma bonds are stronger than pi- bonds. A sigma bond is formed by the overlapping of atomic orbitals along the axis and pi-bond is formed by overlapping of two lobes of the atomic orbitals.
Complete answer:
Ethane is an alkane i.e. the carbon atoms are singly bonded to each other. The formula of ethane is ${{C}_{6}}{{H}_{6}}$.
We know that covalent bonds are formed by sharing of electrons between two atoms. Atoms which do not have their valence shells fulfilled forms covalent bonding with a similar atom to complete its octet and hence gain stability.
Generally, there are three types of covalent bonding that we know about. They are-
Single bond- When there is a sharing of two electrons between a pair of atoms and gives rise to a sigma bond between the atoms, the sigma bond formed is the single bond.
Double bond- When four electrons are shared by the two atoms, it gives rise to a sigma bond and a pi-bond which is known as the double bond.
Triple bond- When six electrons are shared by the two atoms, there exist one sigma and two pi-bonds thus forming a triple bond.
In ethane, we have a carbon-carbon bold and six carbon-hydrogen bonds.
We know that the atomic number of carbon is 6, which means it has 4 electrons in its valence shell. It needs to share 4 electrons to gain stability by completing its octet and atomic number of hydrogen is 1 and it will need 1 electron. The structure of ethane is-

Here, three hydrogen atoms are attached to one carbon atom and the two carbon atoms are bonded to each other too to fulfil their octets. Therefore, all of the bonds present here are covalent and as we can see, there are 7 bonds in total.
Therefore, in ethane there are 7 covalent bonds.
Note:
In order to form a covalent bond, the atoms need to be in a specific arrangement which will allow the overlapping between the orbitals. It is difficult to break a sigma bond because sigma bonds are stronger than pi- bonds. A sigma bond is formed by the overlapping of atomic orbitals along the axis and pi-bond is formed by overlapping of two lobes of the atomic orbitals.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




