
Write the names of product when ethene reacts with ${O_3}$
A.Acetaldehyde
B.Formaldehyde
C.Acetone
D.Formic acid
Answer
577.8k+ views
Hint: We can obtain products such as alcohols, carboxylic acids, aldehydes (or) ketones by the ozonolysis of alkenes.
Ozonolysis is an organic redox reaction that uses ozone to cleave the unsaturated bonds present in alkenes, azo compounds and alkynes.
Complete step by step answer:
We know that ozone is an active allotrope of oxygen. The oxidative cleavage of the alkyne (or) alkene takes place when alkenes and alkynes are reacted with ozone. The carbon-carbon double bond replaces the carbon-carbon triple bonds, and carbonyl products are formed. The carbon-carbon pi bond as well as the carbon-carbon sigma bond is broken by the ozone molecule. An intermediate known as ozonide is formed. We can eliminate the oxygen in the intermediate by the action of zinc dust. Based on the type of reactant and workup, the final product varies.
We can produce aldehydes or ketone when we use thiourea, dimethyl sulfide, zinc dust, triphenylphosphine during the ozonolysis of alkene. The product carboxylic acid is obtained when we use hydrogen peroxide during the ozonolysis of alkene.
Ozonolysis of ethene gives formaldehyde as the product. We can write the chemical reaction as,
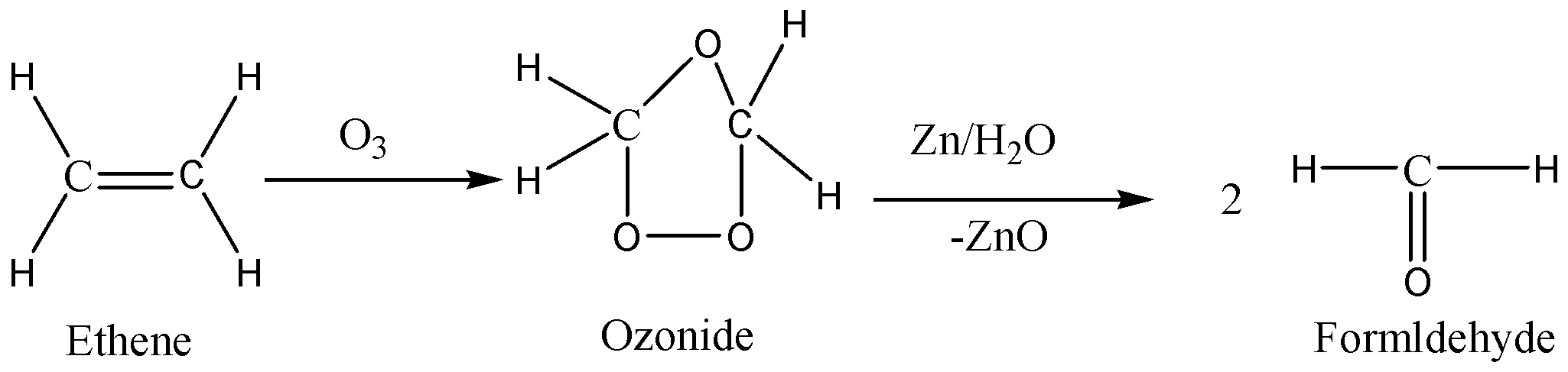
Therefore, the option (B) is correct.
Note:
We must remember that the oleic acid ozonolysis is used to form azelaic acid and pelargonic acid on an industrial scale. Ozonolysis of alkynes gives the product as acid anhydride (or) diketone. Incomplete fragmentation takes place in ozonolysis of alkynes. No reducing agents are used. When the reaction takes place in presence of water, hydrolysis of acid anhydride takes place and two carboxylic acids are formed as products. We can determine the position of triple bonds in an unknown alkyne in ozonolysis of alkynes.
Ozonolysis is an organic redox reaction that uses ozone to cleave the unsaturated bonds present in alkenes, azo compounds and alkynes.
Complete step by step answer:
We know that ozone is an active allotrope of oxygen. The oxidative cleavage of the alkyne (or) alkene takes place when alkenes and alkynes are reacted with ozone. The carbon-carbon double bond replaces the carbon-carbon triple bonds, and carbonyl products are formed. The carbon-carbon pi bond as well as the carbon-carbon sigma bond is broken by the ozone molecule. An intermediate known as ozonide is formed. We can eliminate the oxygen in the intermediate by the action of zinc dust. Based on the type of reactant and workup, the final product varies.
We can produce aldehydes or ketone when we use thiourea, dimethyl sulfide, zinc dust, triphenylphosphine during the ozonolysis of alkene. The product carboxylic acid is obtained when we use hydrogen peroxide during the ozonolysis of alkene.
Ozonolysis of ethene gives formaldehyde as the product. We can write the chemical reaction as,

Therefore, the option (B) is correct.
Note:
We must remember that the oleic acid ozonolysis is used to form azelaic acid and pelargonic acid on an industrial scale. Ozonolysis of alkynes gives the product as acid anhydride (or) diketone. Incomplete fragmentation takes place in ozonolysis of alkynes. No reducing agents are used. When the reaction takes place in presence of water, hydrolysis of acid anhydride takes place and two carboxylic acids are formed as products. We can determine the position of triple bonds in an unknown alkyne in ozonolysis of alkynes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




