
Write the name and structure of any five oxoacids of phosphorus?
Answer
568.2k+ views
Hint:. We know that oxoacids contain oxygen. In the oxoacids, there are different types of bond-like P-OH, P=O bond, and many more. The formation of different oxoacids also depends upon the oxidation state of phosphorus. We can name, and draw the structure of phosphorus.
Complete step by step answer:
-First, we will discuss the bonds found in the oxoacids; as mentioned there is the presence of one $P-OH,\text{or }P=O$ bond. But bonds like P-P or P-H can be also there in consideration with the other bonds.
-We also mentioned the factor oxidation state, then it should be less than +5 for the phosphorus.
-Now, we will draw the structures of five oxoacids of phosphorus.
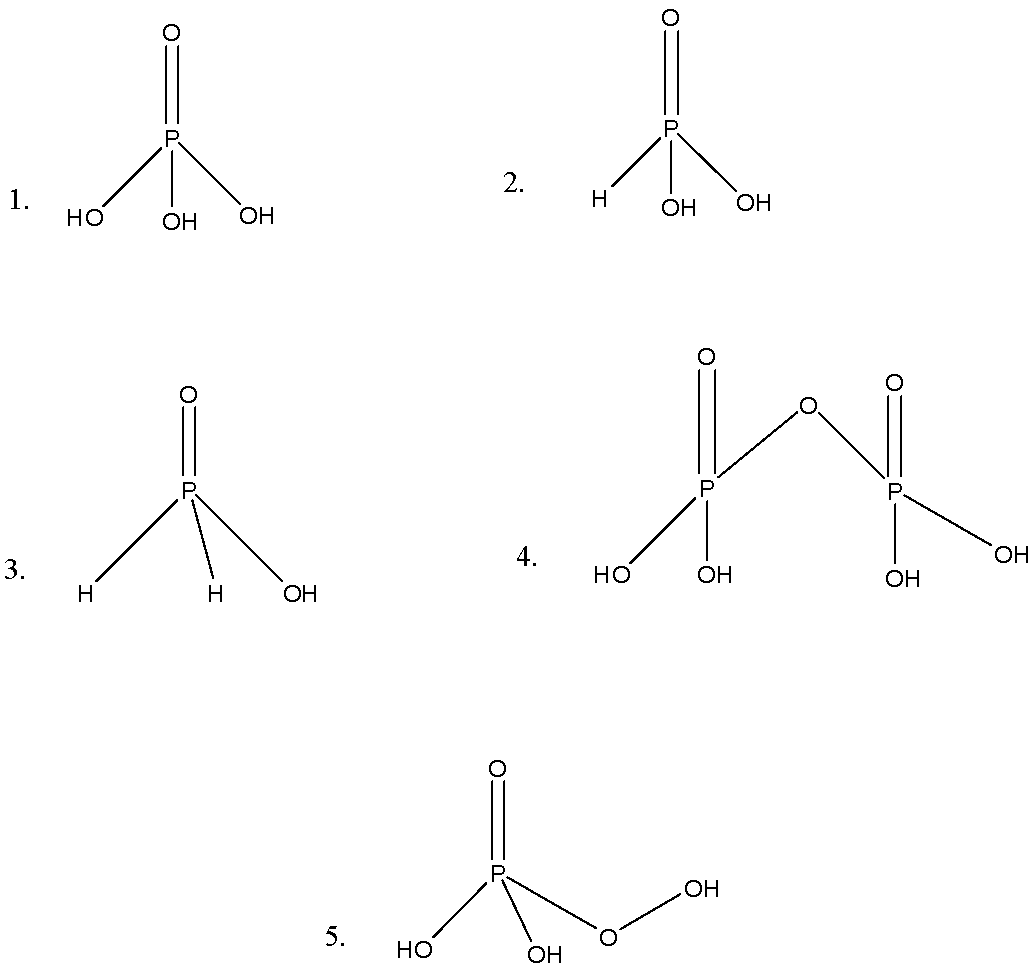
-Now, we can see the five structures. Let us discuss these structures.
-The first we have ${{H}_{3}}P{{O}_{4}}$, in this structure, there are three P-OH bonds and one $P=O$ bond; it represents the tetrahedral representation. It is named as orthophosphoric acid.
-The second structure we have ${{H}_{3}}P{{O}_{3}}$, in this structure we can see P-H bond in addition to the P-OH, and $P=O$ bonds. It is named as orthophosphorous acid.
-The third we have ${{H}_{3}}P{{O}_{2}}$, in this structure we can see the presence of 2P-H bonds, and it is named as hypophosphorous acid.
-The fourth we have ${{H}_{4}}{{P}_{2}}{{O}_{7}}$, in this we can see the presence of P-O-P bond, and it is named as pyrophosphoric acid.
-The last we have ${{H}_{3}}P{{O}_{5}}$, in this structure we can see the P-O-OH bond, or we can say peroxide bond, so it named as polyphosphoric acid.
-In the last, we can conclude that the above mentioned are oxoacids of phosphorus.
Note: Don’t get confused while drawing the structures of the oxoacids of phosphorus, just complete the valency of phosphorus. In all these structures phosphorus depicts the oxidation state less than 5.
Complete step by step answer:
-First, we will discuss the bonds found in the oxoacids; as mentioned there is the presence of one $P-OH,\text{or }P=O$ bond. But bonds like P-P or P-H can be also there in consideration with the other bonds.
-We also mentioned the factor oxidation state, then it should be less than +5 for the phosphorus.
-Now, we will draw the structures of five oxoacids of phosphorus.

-Now, we can see the five structures. Let us discuss these structures.
-The first we have ${{H}_{3}}P{{O}_{4}}$, in this structure, there are three P-OH bonds and one $P=O$ bond; it represents the tetrahedral representation. It is named as orthophosphoric acid.
-The second structure we have ${{H}_{3}}P{{O}_{3}}$, in this structure we can see P-H bond in addition to the P-OH, and $P=O$ bonds. It is named as orthophosphorous acid.
-The third we have ${{H}_{3}}P{{O}_{2}}$, in this structure we can see the presence of 2P-H bonds, and it is named as hypophosphorous acid.
-The fourth we have ${{H}_{4}}{{P}_{2}}{{O}_{7}}$, in this we can see the presence of P-O-P bond, and it is named as pyrophosphoric acid.
-The last we have ${{H}_{3}}P{{O}_{5}}$, in this structure we can see the P-O-OH bond, or we can say peroxide bond, so it named as polyphosphoric acid.
-In the last, we can conclude that the above mentioned are oxoacids of phosphorus.
Note: Don’t get confused while drawing the structures of the oxoacids of phosphorus, just complete the valency of phosphorus. In all these structures phosphorus depicts the oxidation state less than 5.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




