
Write the MO electronic configuration of a diatomic molecule having a bond order of three.
Answer
596.4k+ views
Hint: MO electronic configuration means the molecular orbital electronic configuration of a molecule. As we have to write the MO electronic configuration of diatomic molecules having bond order 3 we will be dealing with nitrogen.
Complete step by step answer:
Molecular electronic configuration depends upon the molecular orbital theory. Molecular orbital theory describes the distribution of electrons in the same way as the distribution of electrons in atoms is defined by using atomic orbitals.
We will consider the molecular orbitals in molecules composed of two identical atoms such as ${{{H}}_{{2}}}$ or ${{C}}{{{l}}_{{2}}}$. Such molecules are known as homonuclear diatomic molecules.
Molecular orbital theory actually uses a linear combination of atomic orbitals to represent molecular orbitals resulting from bonds between atoms. They are generally divided into three types: bonding, antibonding and non-bonding. Common bonding orbitals are sigma orbitals that are symmetric about the bond axis and $\pi$ orbitals with a nodal plane along the bond axis. Antibonding orbitals are shown by the addition of asterisk (*). For example, an antibonding pi orbital may be shown as $\pi^{*}$.
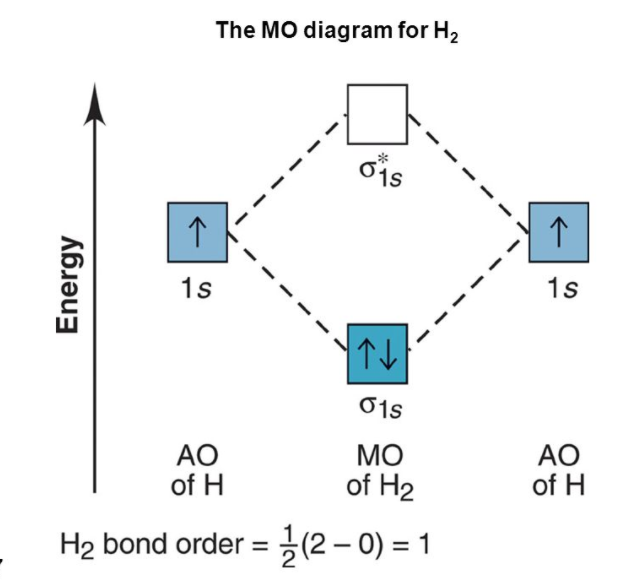
This is the representation of the molecular orbital diagram of molecules.
Now, as according to the question we will deal with the diatomic molecule with bond order ${{3}}$ that is ${{{N}}_{{2}}}$.
The molecular orbitals of ${{{N}}_{{2}}}$ are formed by overlapping the atomic orbitals of the ${{N}}$ atoms.
The new molecular orbitals are formed as:
1. The ${{1s}}$ orbitals form a bonding sigma ${{1s}}$ and an antibonding one.
2. the ${{2s}}$ orbitals form a bonding sigma ${{2s}}$ and are the same as an antibonding one.
3. the ${{2p}}$ orbitals directed along the internuclear axis form a bonding sigma ${{2}}{{{p}}_{{z}}}$ and an antibonding molecular orbital.
4. the ${{2}}{{{p}}_{{x}}}$and ${{2}}{{{p}}_{{y}}}$orbitals from each atom form bonding ${{\pi 2}}{{{p}}_{{x}}}$and ${{\pi 2}}{{{p}}_{{y}}}$bonding molecular orbitals and antibonding ${{{\pi }}^{{*}}}{{2}}{{{p}}_{{x}}}$and ${{{\pi }}^{{*}}}{{2}}{{{p}}_{{y}}}$ molecular orbitals.
Each ${{N}}$atom contributes seven electrons so we use the Aufbau principle to fill the molecular orbitals starting at lowest level.

The above diagram represents the molecular electronic configuration of nitrogen atoms.
Note:
The filling of electrons should be done carefully according to the Aufbau principle. The orbitals should be filled according to that the lowest energy orbital should be filled first and so on.
Complete step by step answer:
Molecular electronic configuration depends upon the molecular orbital theory. Molecular orbital theory describes the distribution of electrons in the same way as the distribution of electrons in atoms is defined by using atomic orbitals.
We will consider the molecular orbitals in molecules composed of two identical atoms such as ${{{H}}_{{2}}}$ or ${{C}}{{{l}}_{{2}}}$. Such molecules are known as homonuclear diatomic molecules.
Molecular orbital theory actually uses a linear combination of atomic orbitals to represent molecular orbitals resulting from bonds between atoms. They are generally divided into three types: bonding, antibonding and non-bonding. Common bonding orbitals are sigma orbitals that are symmetric about the bond axis and $\pi$ orbitals with a nodal plane along the bond axis. Antibonding orbitals are shown by the addition of asterisk (*). For example, an antibonding pi orbital may be shown as $\pi^{*}$.

This is the representation of the molecular orbital diagram of molecules.
Now, as according to the question we will deal with the diatomic molecule with bond order ${{3}}$ that is ${{{N}}_{{2}}}$.
The molecular orbitals of ${{{N}}_{{2}}}$ are formed by overlapping the atomic orbitals of the ${{N}}$ atoms.
The new molecular orbitals are formed as:
1. The ${{1s}}$ orbitals form a bonding sigma ${{1s}}$ and an antibonding one.
2. the ${{2s}}$ orbitals form a bonding sigma ${{2s}}$ and are the same as an antibonding one.
3. the ${{2p}}$ orbitals directed along the internuclear axis form a bonding sigma ${{2}}{{{p}}_{{z}}}$ and an antibonding molecular orbital.
4. the ${{2}}{{{p}}_{{x}}}$and ${{2}}{{{p}}_{{y}}}$orbitals from each atom form bonding ${{\pi 2}}{{{p}}_{{x}}}$and ${{\pi 2}}{{{p}}_{{y}}}$bonding molecular orbitals and antibonding ${{{\pi }}^{{*}}}{{2}}{{{p}}_{{x}}}$and ${{{\pi }}^{{*}}}{{2}}{{{p}}_{{y}}}$ molecular orbitals.
Each ${{N}}$atom contributes seven electrons so we use the Aufbau principle to fill the molecular orbitals starting at lowest level.

The above diagram represents the molecular electronic configuration of nitrogen atoms.
Note:
The filling of electrons should be done carefully according to the Aufbau principle. The orbitals should be filled according to that the lowest energy orbital should be filled first and so on.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




