
Write the Lewis structure of hydrogen peroxide
Answer
563.1k+ views
Hint Lewis dot structure is basically a graphical representation of the electron distribution around an atom. This method helps in predicting the number and type of bonds which can be formed around an atom.
Complete step by step answer:
- Lewis dot structure is very helpful in predicting the geometry of the molecule.
- Hydrogen peroxide is an important chemical compound with the formula $H_{2}O_{2}$.
- When in pure state, hydrogen peroxide has a light blue colour. It is used as an oxidizer, an antiseptic and bleaching agent. Concentrated hydrogen peroxide is a type of a reactive oxygen species that makes it a good propellant in rocketry.
- Hydrogen peroxide has a non-polar, open book like structure with oxygen bond (O-O) spins.
- The bond length of oxygen bond is 145.8 pm and the bond length between O-H is 98.8 pm.
- To obtain the Lewis structure, we will first draw the skeleton structure. Here, the central atom connects all the atoms using a single bond. In this case, one oxygen atom is bonded with other oxygen atom and the form a single bond with hydrogen as shown
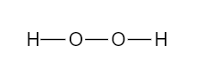
Now, as we can see, 6 electrons are used to make the skeleton structure. We will use the remaining 10 electrons to fill the octet.
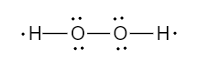
Since, the octets are now filled, the final Lewis dot structure of Hydrogen peroxide is shown below
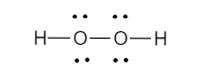
Note: The structure of hydrogen peroxide is non-polar which means it has a three-dimensional quality. When exposed to light, it decomposes. This decomposition process is catalysed by traces of alkali metals.
Complete step by step answer:
- Lewis dot structure is very helpful in predicting the geometry of the molecule.
- Hydrogen peroxide is an important chemical compound with the formula $H_{2}O_{2}$.
- When in pure state, hydrogen peroxide has a light blue colour. It is used as an oxidizer, an antiseptic and bleaching agent. Concentrated hydrogen peroxide is a type of a reactive oxygen species that makes it a good propellant in rocketry.
- Hydrogen peroxide has a non-polar, open book like structure with oxygen bond (O-O) spins.
- The bond length of oxygen bond is 145.8 pm and the bond length between O-H is 98.8 pm.
- To obtain the Lewis structure, we will first draw the skeleton structure. Here, the central atom connects all the atoms using a single bond. In this case, one oxygen atom is bonded with other oxygen atom and the form a single bond with hydrogen as shown
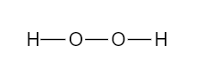
Now, as we can see, 6 electrons are used to make the skeleton structure. We will use the remaining 10 electrons to fill the octet.

Since, the octets are now filled, the final Lewis dot structure of Hydrogen peroxide is shown below

Note: The structure of hydrogen peroxide is non-polar which means it has a three-dimensional quality. When exposed to light, it decomposes. This decomposition process is catalysed by traces of alkali metals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




