
Write the IUPAC name of ${\left( {{\text{C}}{{\text{H}}_{\text{3}}}} \right)_{\text{2}}}{\text{CHCH(Cl)C}}{{\text{H}}_{\text{3}}}$.
Answer
587.1k+ views
Hint: The name of alkane includes all substituents arranged in alphabetical order with their position and the name of the parent chain with the position of the double bond. There are some rules which must be followed by naming an alkane. Let us discuss this.
Complete step by step answer:
The IUPAC rules are used for naming the alkane.
The IUPAC rules for the naming of alkane are as follows:
Determine the longest carbon chain and determine the substituents attached to the chain.
If two same long chains are present then select the more substituted chain.
Give the numbering to the carbon atoms of the chains so all the substituents get the lowest numbering.
Arrange the substituents in alphabetical order with their position in the chain.
If the same substituent is present more than one, then add ‘di’ for two, ‘tri’ for three, and so on.
Select the longest parent chain as follows:
The longest carbon chain is of four carbon atoms. The name of five carbon atoms having alkane is ‘butane.
Determine the substituents as follows:
The longest chain has one chloride group and one methyl group.
Give the numbering to the chain as follows:
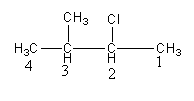
So, the chloride group is present at carbon-2 and the methyl group is present at carbon-3.
Arrange the substituents in alphabetical order so the name of the compound is of 2-chloro-3-methylbutane.
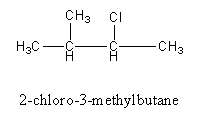
Therefore, the name of the structure is of 2-chloro-3-methylbutane.
Note: In the molecule, the numbering is given from left to right and the substituents are getting the positions and 3. If the numbering is given from right to left, the substituents will get positions 2 and 3 so, from both sides the numbering is the same. In this the numbering is decided on the basis of substituents. According to alphabetical order, chlorine substituents will come first so, it will get the lowest numbering.
Complete step by step answer:
The IUPAC rules are used for naming the alkane.
The IUPAC rules for the naming of alkane are as follows:
Determine the longest carbon chain and determine the substituents attached to the chain.
If two same long chains are present then select the more substituted chain.
Give the numbering to the carbon atoms of the chains so all the substituents get the lowest numbering.
Arrange the substituents in alphabetical order with their position in the chain.
If the same substituent is present more than one, then add ‘di’ for two, ‘tri’ for three, and so on.
Select the longest parent chain as follows:
The longest carbon chain is of four carbon atoms. The name of five carbon atoms having alkane is ‘butane.
Determine the substituents as follows:
The longest chain has one chloride group and one methyl group.
Give the numbering to the chain as follows:

So, the chloride group is present at carbon-2 and the methyl group is present at carbon-3.
Arrange the substituents in alphabetical order so the name of the compound is of 2-chloro-3-methylbutane.

Therefore, the name of the structure is of 2-chloro-3-methylbutane.
Note: In the molecule, the numbering is given from left to right and the substituents are getting the positions and 3. If the numbering is given from right to left, the substituents will get positions 2 and 3 so, from both sides the numbering is the same. In this the numbering is decided on the basis of substituents. According to alphabetical order, chlorine substituents will come first so, it will get the lowest numbering.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




