
Write the IUPAC name of $C{H_3}.C.({C_2}{H_5}).C{H_2}Br$
Answer
573.3k+ views
Hint: In the given compound, the central atom is the carbon. The carbon has four valence electrons that means carbon can make four chemical bonds with four atoms by sharing its four electrons.
Complete step by step answer:
The International Union of Pure and Applied Chemistry (IUPAC) have confirmed some guidelines for the naming of chemical compounds.
The given compound is shown below.
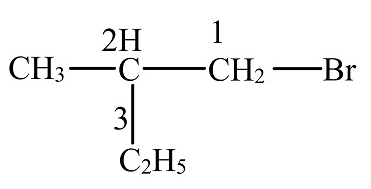
The above diagram is drawn using adobe flash.
STEP 1: Identify the functional group or other group
The compound is the aliphatic hydrocarbon. In the first position of the alkane chain, bromine group is attached.
STEP 2: Find the longest carbon chain
The longest carbon chain consists of four carbon atoms, therefore the hydrocarbon is butane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering is done from the position where the halogen group is attached.
STEP 4: Look for any branched molecule attached to the chain
In the given compound, one methyl group is attached to the second position of the chain.
STEP 5: Combine the entire element into a single IUPAC name
So, the name of the given compound is 1-bromo-2-methyl-butane.
Note: In the given compound hydrogen atom is not shown but the covalency of carbon is four, so it is attached to four other atoms to fulfill its valency. The parent chain is determined as the one which contains the highest number of carbon atoms, as the ethyl group is attached to the second carbon, therefore, the hydrocarbon is said to be butane as total carbon in the chain is four.
Complete step by step answer:
The International Union of Pure and Applied Chemistry (IUPAC) have confirmed some guidelines for the naming of chemical compounds.
The given compound is shown below.

The above diagram is drawn using adobe flash.
STEP 1: Identify the functional group or other group
The compound is the aliphatic hydrocarbon. In the first position of the alkane chain, bromine group is attached.
STEP 2: Find the longest carbon chain
The longest carbon chain consists of four carbon atoms, therefore the hydrocarbon is butane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering is done from the position where the halogen group is attached.
STEP 4: Look for any branched molecule attached to the chain
In the given compound, one methyl group is attached to the second position of the chain.
STEP 5: Combine the entire element into a single IUPAC name
So, the name of the given compound is 1-bromo-2-methyl-butane.
Note: In the given compound hydrogen atom is not shown but the covalency of carbon is four, so it is attached to four other atoms to fulfill its valency. The parent chain is determined as the one which contains the highest number of carbon atoms, as the ethyl group is attached to the second carbon, therefore, the hydrocarbon is said to be butane as total carbon in the chain is four.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




