
Write the formula of allyl alcohol and benzyl alcohol.
Answer
591.6k+ views
Hint:
The IUPAC name for allyl alcohol is 2-propen-1-ol.
The IUPAC name for benzyl alcohol is phenylmethanol.
Complete step by step answer:
-Step 1:
-Write the condensed structural formula of allyl alcohol as follows:
-The IUPAC name for allyl alcohol is 2-propen-1-ol. 2-propen suggests that there are three carbon atoms and double bonds at the second carbon. 1-ol suggests that the $ - {\text{OH}}$ functional group is attached to the first carbon. Thus, the condensed structural formula of allyl alcohol is,
${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of allyl alcohol as follows:
-The structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of allyl alcohol as follows:
The condensed structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains three carbon atoms, six hydrogen atoms and one oxygen atom. Thus, the molecular formula of allyl alcohol is,
${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
-Step 2:
-Write the condensed structural formula of benzyl alcohol as follows:
-The IUPAC name for benzyl alcohol is phenylmethanol. Phenylmethanol suggests that methanol${\text{C}}{{\text{H}}_2} - {\text{OH}}$ functional group is attached to the phenyl ring. Thus, the condensed structural formula of benzyl alcohol is,
${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of benzyl alcohol as follows:
-The structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.
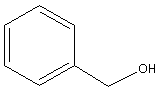
-Write the molecular formula of benzyl alcohol as follows:
-The condensed structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains seven carbon atoms, eight hydrogen atoms and one oxygen atom. Thus, the molecular formula of benzyl alcohol is,
${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}{\text{O}}$.
Note: A molecule is represented using a molecular formula. The molecular formula represents the number of atoms of different elements present in molecules.
The structural formula represents which atoms are bonded to each other and the condensed structural formula is condensed form of structural formula.
The IUPAC name for allyl alcohol is 2-propen-1-ol.
The IUPAC name for benzyl alcohol is phenylmethanol.
Complete step by step answer:
-Step 1:
-Write the condensed structural formula of allyl alcohol as follows:
-The IUPAC name for allyl alcohol is 2-propen-1-ol. 2-propen suggests that there are three carbon atoms and double bonds at the second carbon. 1-ol suggests that the $ - {\text{OH}}$ functional group is attached to the first carbon. Thus, the condensed structural formula of allyl alcohol is,
${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of allyl alcohol as follows:
-The structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of allyl alcohol as follows:
The condensed structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains three carbon atoms, six hydrogen atoms and one oxygen atom. Thus, the molecular formula of allyl alcohol is,
${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
-Step 2:
-Write the condensed structural formula of benzyl alcohol as follows:
-The IUPAC name for benzyl alcohol is phenylmethanol. Phenylmethanol suggests that methanol${\text{C}}{{\text{H}}_2} - {\text{OH}}$ functional group is attached to the phenyl ring. Thus, the condensed structural formula of benzyl alcohol is,
${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of benzyl alcohol as follows:
-The structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of benzyl alcohol as follows:
-The condensed structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains seven carbon atoms, eight hydrogen atoms and one oxygen atom. Thus, the molecular formula of benzyl alcohol is,
${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}{\text{O}}$.
Note: A molecule is represented using a molecular formula. The molecular formula represents the number of atoms of different elements present in molecules.
The structural formula represents which atoms are bonded to each other and the condensed structural formula is condensed form of structural formula.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




