
How do you write the electron dot diagram for the hydroxide ion $O{H^ - }$?
Answer
564.9k+ views
Hint: For writing the electron dot diagram of the hydroxide ion, we have to know about the electrons which are present in the outermost shell of each atom involves in the molecule and formal charge of the molecule is present on which atom.
Complete answer:
Some points which we have to know before constructing the electron dot structure of hydroxide ion are as follow:
-Atomic number of oxygen atom is eight and its electronic configuration is written as $1{s^2},2{s^2}2{p^4}$ and from this electronic configuration it is clear that in the outermost shell of oxygen six valence electrons are present.
-Atomic number of hydrogen atoms is one and its electronic configuration is written as $1{s^1}$ and from this electronic configuration it is clear that in the outermost shell of hydrogen one valence electron is present.
-In the hydroxide ion negative charge is present on the molecule, but this negative charge is actually present on the oxygen atom and due to this negative charge one extra electron is present on the outermost shell of the oxygen i.e. in place of six electrons seven electrons are present as valence electrons on the outermost shell.
So, the electron dot diagram of the hydroxide ion is as follow:
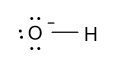
In the above diagram it is clear that out of seven electrons of oxygen atom six electrons are shown by the dots and the seventh electron is involved in the formation of bond with one electron of the hydrogen atom.
Note:
Here some of you may think that the negative charge of the molecule is not bear by the hydrogen atom, so the reason is that oxygen is electronegative in nature that’s why it has a tendency to attract electrons from outside.
Complete answer:
Some points which we have to know before constructing the electron dot structure of hydroxide ion are as follow:
-Atomic number of oxygen atom is eight and its electronic configuration is written as $1{s^2},2{s^2}2{p^4}$ and from this electronic configuration it is clear that in the outermost shell of oxygen six valence electrons are present.
-Atomic number of hydrogen atoms is one and its electronic configuration is written as $1{s^1}$ and from this electronic configuration it is clear that in the outermost shell of hydrogen one valence electron is present.
-In the hydroxide ion negative charge is present on the molecule, but this negative charge is actually present on the oxygen atom and due to this negative charge one extra electron is present on the outermost shell of the oxygen i.e. in place of six electrons seven electrons are present as valence electrons on the outermost shell.
So, the electron dot diagram of the hydroxide ion is as follow:
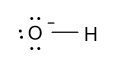
In the above diagram it is clear that out of seven electrons of oxygen atom six electrons are shown by the dots and the seventh electron is involved in the formation of bond with one electron of the hydrogen atom.
Note:
Here some of you may think that the negative charge of the molecule is not bear by the hydrogen atom, so the reason is that oxygen is electronegative in nature that’s why it has a tendency to attract electrons from outside.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




