
Write the chemical reaction to prepare novolac polymer.
Answer
584.7k+ views
Hint: The large molecules made when many smaller molecules are joined together end to end are called polymers. The smaller repeating molecules are called the monomers and the process of making a polymer from its monomers is known as polymerization.
Complete answer: or Complete step by step answer: Novolac is a low molecular weight polymer that is polymerized from phenol and formaldehyde.
As this polymer is being derived from two different monomers, therefore, it is known as a copolymer and the process of formation or polymerization of such polymers, made from different monomers is known as copolymerization.
Novolac is also known as a phenol formaldehyde resin.
The polymerization reaction of novolac can be written as:
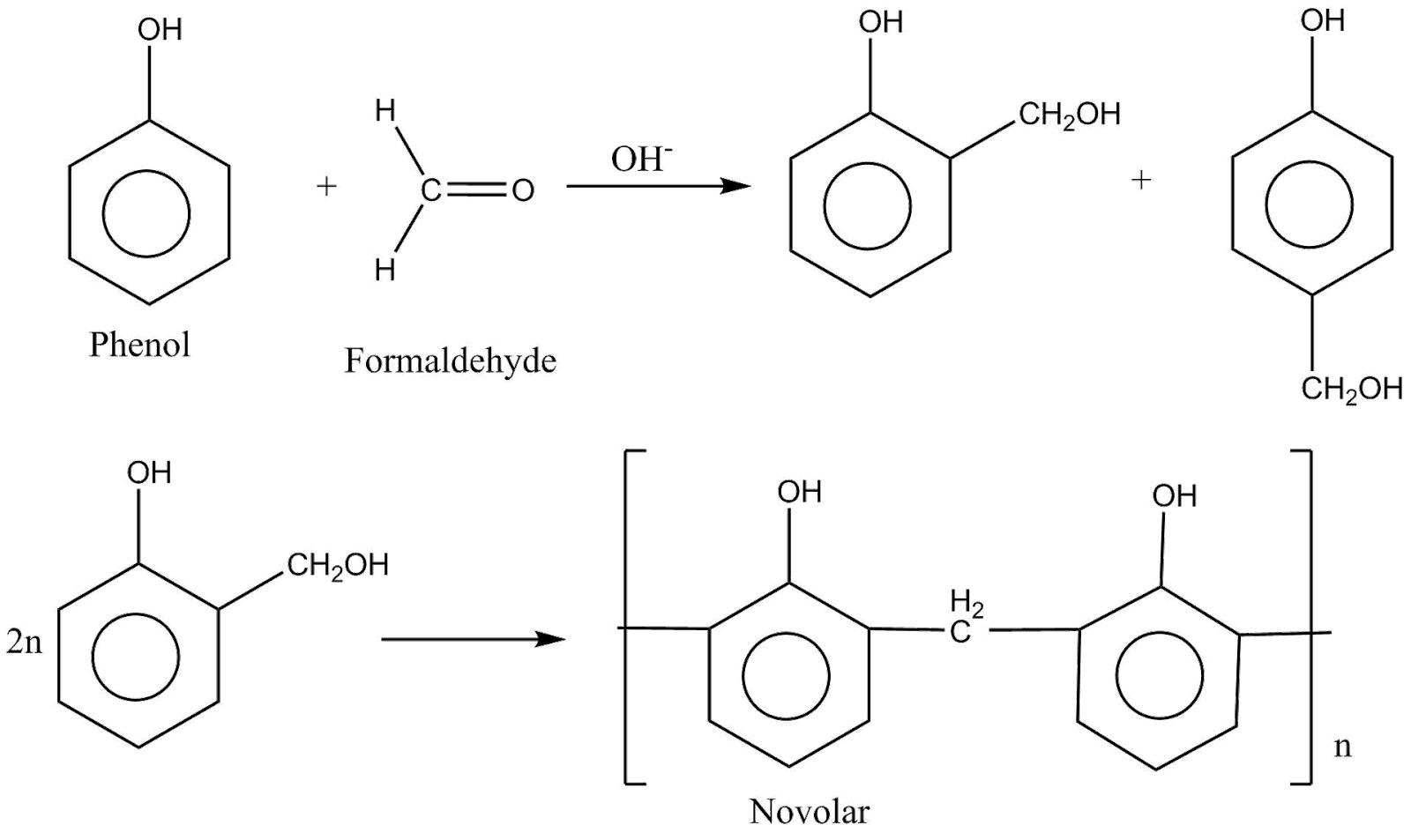
The above reaction can be called a stop growth polymerization reaction which could either be acid or base catalysed.
The phenol reacts with formaldehyde at the ortho and para positions and leads to the formation of hydroxymethyl phenol. The hydroxymethyl group is capable of reacting with either another free ortho or para group or with another hydroxymethyl group. Now, this leads to the formation of a linear polymer called novolac.
Note: Novolac is a resin which is produced from the complete poly-condensation of phenol and aldehydes for industrial uses as a replacement of the natural resin.
Complete answer: or Complete step by step answer: Novolac is a low molecular weight polymer that is polymerized from phenol and formaldehyde.
As this polymer is being derived from two different monomers, therefore, it is known as a copolymer and the process of formation or polymerization of such polymers, made from different monomers is known as copolymerization.
Novolac is also known as a phenol formaldehyde resin.
The polymerization reaction of novolac can be written as:

The above reaction can be called a stop growth polymerization reaction which could either be acid or base catalysed.
The phenol reacts with formaldehyde at the ortho and para positions and leads to the formation of hydroxymethyl phenol. The hydroxymethyl group is capable of reacting with either another free ortho or para group or with another hydroxymethyl group. Now, this leads to the formation of a linear polymer called novolac.
Note: Novolac is a resin which is produced from the complete poly-condensation of phenol and aldehydes for industrial uses as a replacement of the natural resin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




