
Write the chemical equation of the following reaction:
(a) Swarts reaction
(b) Sandmeyers reaction
Answer
542.1k+ views
Hint: Generally alkyl fluorides are going to prepare from alkyl chlorides using Swarts reaction. Aryl halides are prepared from benzene diazonium chloride using copper salt, this reaction is called Sanmeyers reaction.
Complete answer:
- In the question it is given to write about the Swarts reaction and Sandmeyers reaction.
Swarts reaction:
Alkyl bromide or alkyl chlorides are going to react with metallic fluorides like AgF, $Co{{F}_{2}},H{{g}_{2}}{{F}_{2}}$ and gives respective alkyl halide as the product.

In the above chemical reaction ethyl chloride reacts with silver fluoride and forms ethyl fluoride and silver chloride as the products.
Sandmeyers reaction: This chemical reaction is named after Traugott Sandmeyer.
It is an example for radical nucleophilic substitution reactions. Here copper salts are going to act as catalysts.
Benzene diazonium chloride is going to react with cuprous halides in the presence of an acid and forms aryl halides as the products.
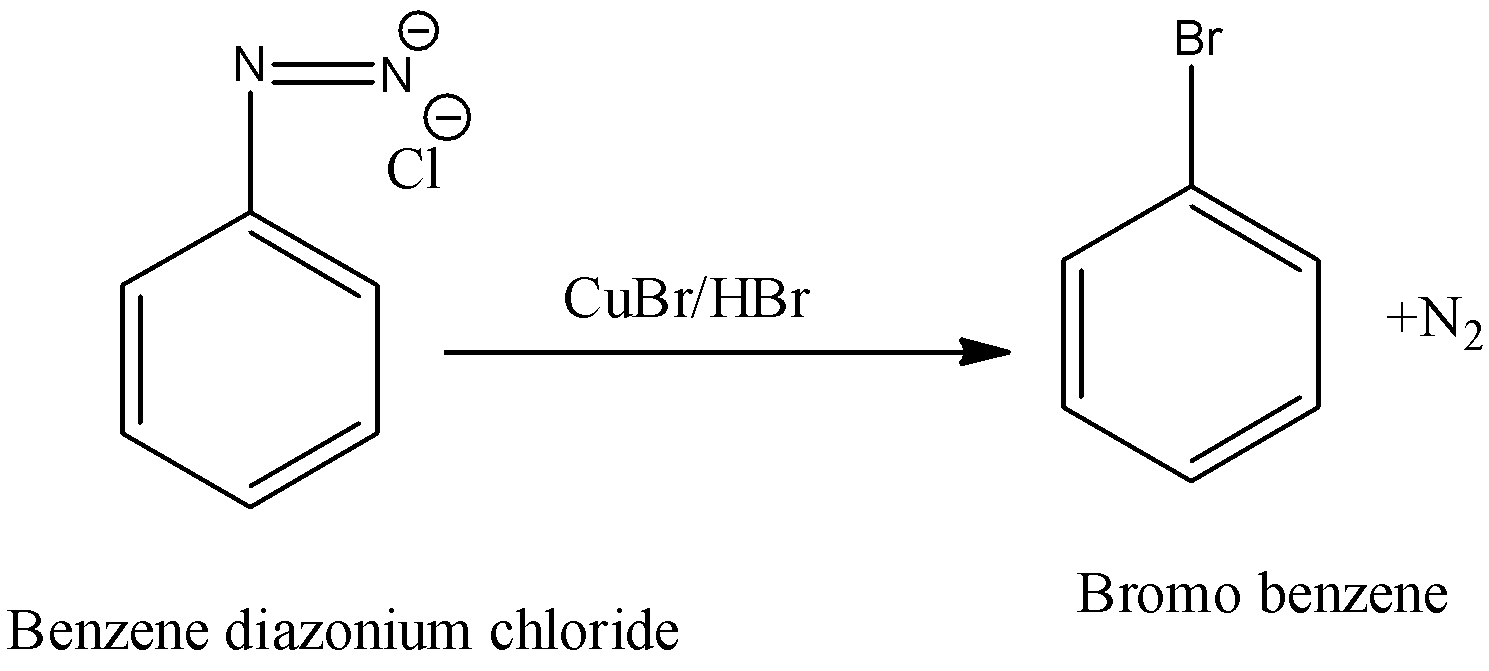
In this chemical reaction we have to use freshly prepared benzene diazonium chloride to carry out the reaction.
In the above chemical reaction benzene diazonium chloride reacts with copper bromide (I) in the presence of HBr and forms bromo benzene and nitrogen gas are the products.
Note:
In industry Swarts reaction and sandmeyer's reactions have a lot of applications to prepare various products. These reactions are called named reactions. By using these two reactions a variety of halides are going to get prepared.
Complete answer:
- In the question it is given to write about the Swarts reaction and Sandmeyers reaction.
Swarts reaction:
Alkyl bromide or alkyl chlorides are going to react with metallic fluorides like AgF, $Co{{F}_{2}},H{{g}_{2}}{{F}_{2}}$ and gives respective alkyl halide as the product.

In the above chemical reaction ethyl chloride reacts with silver fluoride and forms ethyl fluoride and silver chloride as the products.
Sandmeyers reaction: This chemical reaction is named after Traugott Sandmeyer.
It is an example for radical nucleophilic substitution reactions. Here copper salts are going to act as catalysts.
Benzene diazonium chloride is going to react with cuprous halides in the presence of an acid and forms aryl halides as the products.

In this chemical reaction we have to use freshly prepared benzene diazonium chloride to carry out the reaction.
In the above chemical reaction benzene diazonium chloride reacts with copper bromide (I) in the presence of HBr and forms bromo benzene and nitrogen gas are the products.
Note:
In industry Swarts reaction and sandmeyer's reactions have a lot of applications to prepare various products. These reactions are called named reactions. By using these two reactions a variety of halides are going to get prepared.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




