
Write structures of the compounds whose IUPAC names are as follows:
i) 2-Methylbutan-2-ol
ii) 1-phenylpropan-2-ol
iii) 3,5-dimethylhexane 1,35-triol
iv) 2,3-Diethylphenol
v) 1-ethoxypropane
vi) 2-ethoxy-3-methylpentane
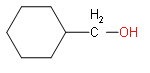
vii) cyclohexylmethanol
viii) 3-cyclohexyl pentan-3-ol
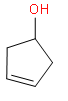
ix) Cyclopent-3-en-1-ol
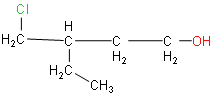
x) 4-chloro-3-ethylbutan-1-ol
Answer
599.1k+ views
Hint: The important point you need to care about while writing the structure of an organic compound is the root word. The root word will give you the longest carbon chain. Then attach the substituents according to their positions given in the name.
Complete step by step answer:
(i) The word root is butane. A hydroxyl group is attached at the second position of butane and one methyl group in the same position.
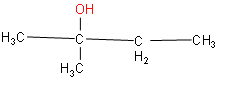
(ii) The root word is propane. One phenyl group is attached in the first position and hydroxyl group in the second position.
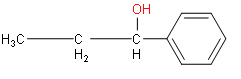
(iii) The word root is hexane. Two methyl groups and three hydroxyl groups are the substituents.
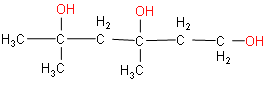
(iv) The root word is phenol and two methyl groups are substituents.
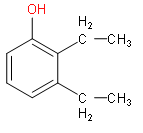
(v) This is an ether. Propane and ethane are sandwiched with oxygen.

(vi) The root word is pentane. One ethoxy group is attached at the second position.

(vii) The root word is methane. One cyclohexane and hydroxyl group is attached.
(viii) The root word is pentane. A cyclohexane group and hydroxyl groups are substituents.

(ix) The root word is cyclopentane. There is a double in the third position and hydroxyl group in the first position.
(x) The root word is butane. Chlorine, ethyl and hydroxyl groups are substituents.
Note: When you are numbering a longest carbon chain, make sure the functional groups should get the least number. While there is more than one functional group, the functional group with the highest priority should get the least number. Carboxylic acid groups have a higher priority than hydroxyl groups.
Complete step by step answer:
(i) The word root is butane. A hydroxyl group is attached at the second position of butane and one methyl group in the same position.

(ii) The root word is propane. One phenyl group is attached in the first position and hydroxyl group in the second position.

(iii) The word root is hexane. Two methyl groups and three hydroxyl groups are the substituents.

(iv) The root word is phenol and two methyl groups are substituents.

(v) This is an ether. Propane and ethane are sandwiched with oxygen.

(vi) The root word is pentane. One ethoxy group is attached at the second position.

(vii) The root word is methane. One cyclohexane and hydroxyl group is attached.

(viii) The root word is pentane. A cyclohexane group and hydroxyl groups are substituents.

(ix) The root word is cyclopentane. There is a double in the third position and hydroxyl group in the first position.

(x) The root word is butane. Chlorine, ethyl and hydroxyl groups are substituents.

Note: When you are numbering a longest carbon chain, make sure the functional groups should get the least number. While there is more than one functional group, the functional group with the highest priority should get the least number. Carboxylic acid groups have a higher priority than hydroxyl groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




