
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) Pent-\[2\]-ene
(ii) \[3,4\]-Dimethylhept-\[3\]-ene
(iii) \[2\]-Ethylbut-\[1\]-ene
(iv) \[1\]-Phenylbut-\[1\]-ene
Answer
569.4k+ views
Hint: Ozonolysis is an organic chemical reaction in which an alkene reacts with ozone and undergoes a bond cleavage reaction. A variety of products are obtained during ozonolysis reactions.
Complete step by step answer:
Ozonolysis is the summation of two words ozone and lysis. This means ozone is used in this reaction as a reagent. The lysis means that the reaction undergoes a cleavage reaction which is known as bond breaking reaction. The reaction is shown as:
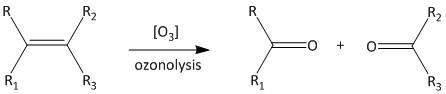
A number of products can be formed with ozonolysis such as alcohols, aldehydes, ketones or carboxylic acids. The cleavage can be predicted as addition of oxygen atom to either side of the double bonded carbon. IUPAC refers to the International Union of Pure and Applied Chemistry. It gives a worldwide method for naming of organic compounds.
Let us determine the product and their IUPAC name after ozonolysis of the given starting materials.
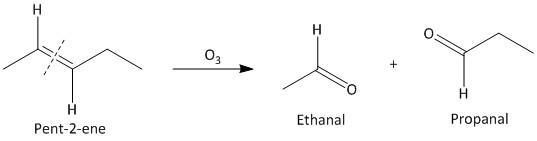
(i) Pent-\[2\]-ene. The ozonolysis of pent-\[2\]-ene leads to formation of two aldehydes ethanal and propanal. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms has hydrogen atoms as substituent attached. The reaction is shown as:
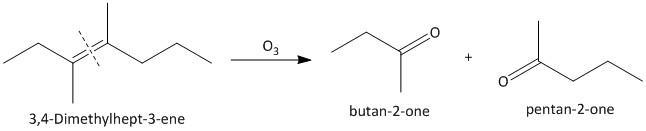
(ii) \[3,4\]-Dimethylhept-\[3\]-ene. The ozonolysis of \[3,4\]-Dimethylhept-\[3\]-ene leads to formation of two ketones as butane-\[2\]-one and pentan-\[2\]-one . The cleavage of the double bond gives two ketones as the double bonded carbon atoms are tetra substituted. The reaction is shown as:
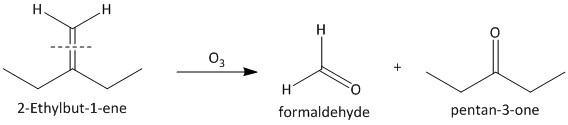
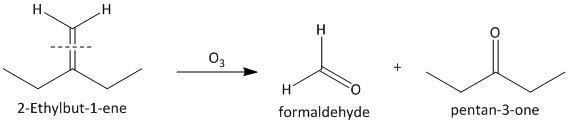
(iii) \[2\]-Ethylbut-\[1\]-ene. The ozonolysis of \[2\]-ethylbut-\[1\]-ene leads to formation of one aldehyde formaldehyde and a ketone. The cleavage of the double bond gives one aldehyde as one of the double bonded carbon atoms has two hydrogen atoms as substituent attached. The reaction is shown as:
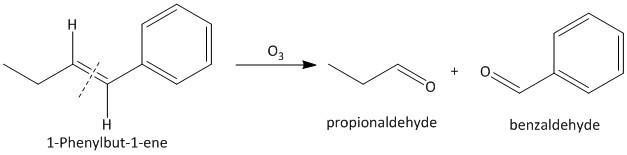
(iv) \[1\]-Phenylbut-\[1\]-ene. The ozonolysis of \[1\]-phenylbut-\[1\]-ene leads to formation of two aldehydes as propionaldehyde and benzaldehyde. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms have two hydrogen atoms as substituent attached. The reaction is shown as:
(Source: The image is drawn in chemdraw.)
(iv) \[1\]-Phenylbut-\[1\]-ene. The ozonolysis of \[1\]-phenylbut-\[1\]-ene leads to formation of two aldehydes as propionaldehyde and benzaldehyde. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms have two hydrogen atoms as substituent attached. The reaction is shown as:
Note:
Ozonolysis reaction is also applicable in case of alkynes and azo compounds. The products obtained from alkenes and alkynes are carbonyl compounds and the product obtained from azo compounds is nitrosamines.
Complete step by step answer:
Ozonolysis is the summation of two words ozone and lysis. This means ozone is used in this reaction as a reagent. The lysis means that the reaction undergoes a cleavage reaction which is known as bond breaking reaction. The reaction is shown as:

A number of products can be formed with ozonolysis such as alcohols, aldehydes, ketones or carboxylic acids. The cleavage can be predicted as addition of oxygen atom to either side of the double bonded carbon. IUPAC refers to the International Union of Pure and Applied Chemistry. It gives a worldwide method for naming of organic compounds.
Let us determine the product and their IUPAC name after ozonolysis of the given starting materials.
(i) Pent-\[2\]-ene. The ozonolysis of pent-\[2\]-ene leads to formation of two aldehydes ethanal and propanal. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms has hydrogen atoms as substituent attached. The reaction is shown as:

(ii) \[3,4\]-Dimethylhept-\[3\]-ene. The ozonolysis of \[3,4\]-Dimethylhept-\[3\]-ene leads to formation of two ketones as butane-\[2\]-one and pentan-\[2\]-one . The cleavage of the double bond gives two ketones as the double bonded carbon atoms are tetra substituted. The reaction is shown as:

(iii) \[2\]-Ethylbut-\[1\]-ene. The ozonolysis of \[2\]-ethylbut-\[1\]-ene leads to formation of one aldehyde formaldehyde and a ketone. The cleavage of the double bond gives one aldehyde as one of the double bonded carbon atoms has two hydrogen atoms as substituent attached. The reaction is shown as:

(iv) \[1\]-Phenylbut-\[1\]-ene. The ozonolysis of \[1\]-phenylbut-\[1\]-ene leads to formation of two aldehydes as propionaldehyde and benzaldehyde. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms have two hydrogen atoms as substituent attached. The reaction is shown as:

(Source: The image is drawn in chemdraw.)
(iv) \[1\]-Phenylbut-\[1\]-ene. The ozonolysis of \[1\]-phenylbut-\[1\]-ene leads to formation of two aldehydes as propionaldehyde and benzaldehyde. The cleavage of the double bond gives two aldehydes as the double bonded carbon atoms have two hydrogen atoms as substituent attached. The reaction is shown as:

Note:
Ozonolysis reaction is also applicable in case of alkynes and azo compounds. The products obtained from alkenes and alkynes are carbonyl compounds and the product obtained from azo compounds is nitrosamines.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




