
Write a short note on the following
Raschig–Hooker process.
Answer
524.1k+ views
Hint: As we know that Raschig–Hooker process is an organic synthesis used for the production of phenol. It is more preferable over Dow’s and Bayer’s process but this process takes place at very high temperatures in a very acidic medium or environment. So here we have to explain the Raschig–Hooker process.
Complete answer:
Let us discuss about Raschig–Hooker process as follows:-
-Raschig–Hooker process: It is a chemical process in organic chemistry which is used for the production of phenol. This process is mainly preferred in European countries. The main steps in this process are as follows:-
(a) Firstly we need to produce chlorobenzene from benzene by using hydrochloric acid (HCl) and oxygen gas. This step uses either a copper or iron chloride catalyst and also exposes these materials to air at 400$^{\circ }C$. This reaction is shown below:-
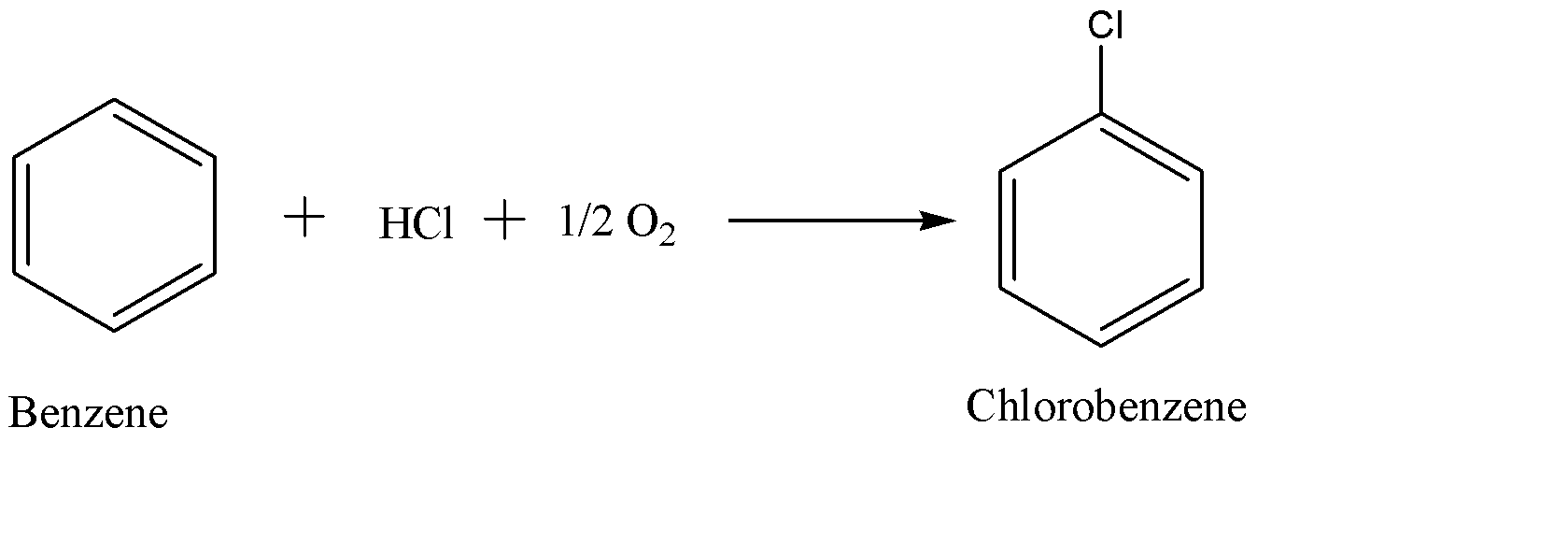
(b) Then chlorobenzene is introduced to steam or water vapour at 450$^{\circ }C$ over a silicon catalyst which hydrolyses the chlorobenzene. This hydrolysis of chlorobenzene yields phenol and hydrogen chloride which can be recycled back to get reused in the first step.
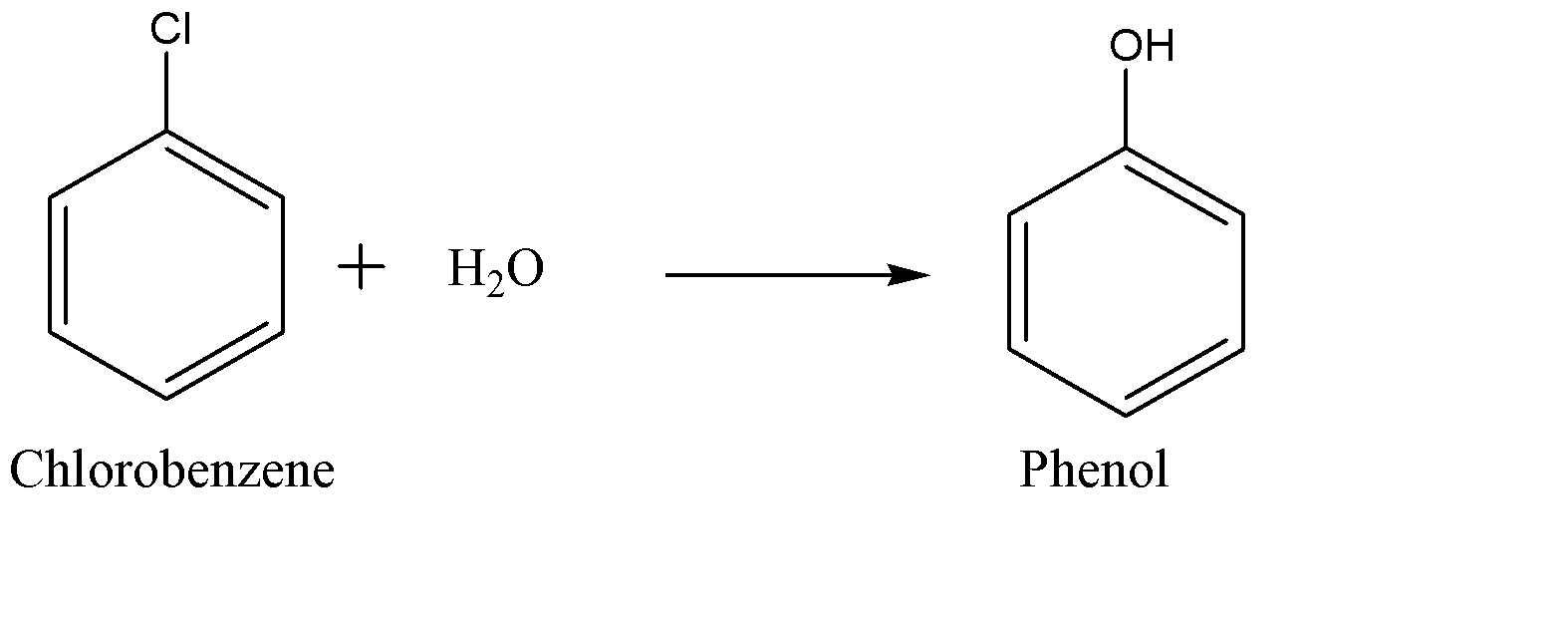
-This whole process takes place at a very high temperature in the acidic medium. The recycling of hydrogen chloride in the end of the process made it more preferable synthesis over Dow’s and Bayer’s process.
Note:
-Remember that there are two steps in the Raschig–Hooker process due to which it can be used to produce either chlorobenzene or phenol.
-Also the overall selectivity of the reaction is $70\%$ to $80\%$ as the second step shares the low conversion rate and the first step has a high selectivity rate.
Complete answer:
Let us discuss about Raschig–Hooker process as follows:-
-Raschig–Hooker process: It is a chemical process in organic chemistry which is used for the production of phenol. This process is mainly preferred in European countries. The main steps in this process are as follows:-
(a) Firstly we need to produce chlorobenzene from benzene by using hydrochloric acid (HCl) and oxygen gas. This step uses either a copper or iron chloride catalyst and also exposes these materials to air at 400$^{\circ }C$. This reaction is shown below:-

(b) Then chlorobenzene is introduced to steam or water vapour at 450$^{\circ }C$ over a silicon catalyst which hydrolyses the chlorobenzene. This hydrolysis of chlorobenzene yields phenol and hydrogen chloride which can be recycled back to get reused in the first step.

-This whole process takes place at a very high temperature in the acidic medium. The recycling of hydrogen chloride in the end of the process made it more preferable synthesis over Dow’s and Bayer’s process.
Note:
-Remember that there are two steps in the Raschig–Hooker process due to which it can be used to produce either chlorobenzene or phenol.
-Also the overall selectivity of the reaction is $70\%$ to $80\%$ as the second step shares the low conversion rate and the first step has a high selectivity rate.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




