
Write a note on vulcanization of rubber.
Answer
591.3k+ views
Hint: We know that rubber is formed as a result of addition polymerization of isoprene. Rubber is vulcanized with sulfur to make the rubber more thermostable and elastic.
Complete step by step answer:
It is already known that polymers are the large molecules formed from the small repeating units. The repeating units are called monomers. The process of formation of polymers from monomer units is called polymerization. Polymers of different masses and different properties are formed. Natural rubber is a polymer with the chemical name polyisoprene. Its monomer unit is isoprene.
We know that condensation polymerization is also known as step growth polymerization; it emits small molecules as a byproduct of polymerization reaction. Addition polymerization is also known as chain-growth polymerization; the monomer units combine without formation of any kind of byproducts with the help of reaction initiators.
Vulcanization is the process of introducing sulfur atoms cross-links between the polymeric chains. It is known to improve the thermo-stability and elasticity of the natural rubber. The stiffness of vulcanized rubber depends on the quantity of added sulphur. Higher tensile strength, increasing of elastic strength to a greater range of temperature, resistance to swelling, and abrasion are the improved properties obtained after vulcanization.
When rubber is treated with sulphur, cross-links formed between polyisoprene units as shown below.
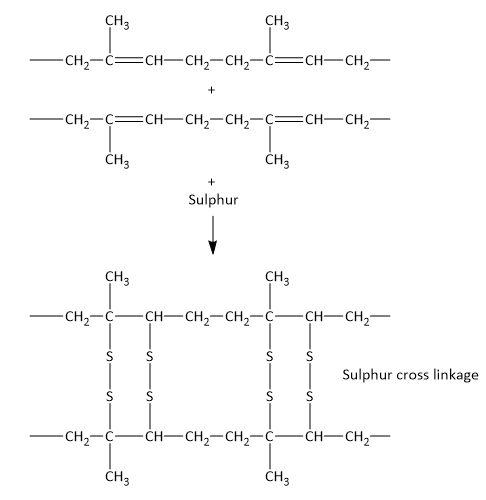
Note:
The polymer after vulcanization has good tensile strength, higher resistance to oxidation, and acts as an improved electrical insulator. It is also found to be resistant to fats, oils and organic solvents.
Complete step by step answer:
It is already known that polymers are the large molecules formed from the small repeating units. The repeating units are called monomers. The process of formation of polymers from monomer units is called polymerization. Polymers of different masses and different properties are formed. Natural rubber is a polymer with the chemical name polyisoprene. Its monomer unit is isoprene.
We know that condensation polymerization is also known as step growth polymerization; it emits small molecules as a byproduct of polymerization reaction. Addition polymerization is also known as chain-growth polymerization; the monomer units combine without formation of any kind of byproducts with the help of reaction initiators.
Vulcanization is the process of introducing sulfur atoms cross-links between the polymeric chains. It is known to improve the thermo-stability and elasticity of the natural rubber. The stiffness of vulcanized rubber depends on the quantity of added sulphur. Higher tensile strength, increasing of elastic strength to a greater range of temperature, resistance to swelling, and abrasion are the improved properties obtained after vulcanization.
When rubber is treated with sulphur, cross-links formed between polyisoprene units as shown below.

Note:
The polymer after vulcanization has good tensile strength, higher resistance to oxidation, and acts as an improved electrical insulator. It is also found to be resistant to fats, oils and organic solvents.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




