
Which will form two oximes with $NH_2OH$ ?
A. $C{H_3}CHO$
B. $C{H_3}COC{H_2}C{H_3}$
C. $HCHO$
D. $C{H_3}COC{H_2}C{H_2}C{H_3}$
Answer
569.4k+ views
Hint: We must remember that the oximes are formed by the reaction hydroxylamine with aldehyde or ketone. We have to remember that the two isomeric oximes are possible with aldehydes (except formaldehydes) and non-symmetric ketones. Oximes is the class of nitrogen containing organic compounds which are generally prepared from hydroxylamine, aldehyde, ketones or Quinone.
Complete step by step answer:
Let’s start with discussing a little about oximes for better understanding of the question. Oximes is the class of nitrogen containing organic compounds which are generally prepared from hydroxylamine, aldehyde, ketones or Quinone. Oximes have a general molecular formula $RR^'$C=NO.
Where, R-alkyl group
R’-hydrogen
Coming back to the question we are asked for the molecule out of the options given to us which will form two oximes with $NH_2OH$. So, in this question we are provided with aldehydes and ketones as the option. Two isomeric oximes are possible with aldehydes (except formaldehydes) and non-symmetric ketones. Out of all the options given there is only one which fulfils the above condition and is capable of forming two isomeric oximes which is $C{H_3}CHO$.
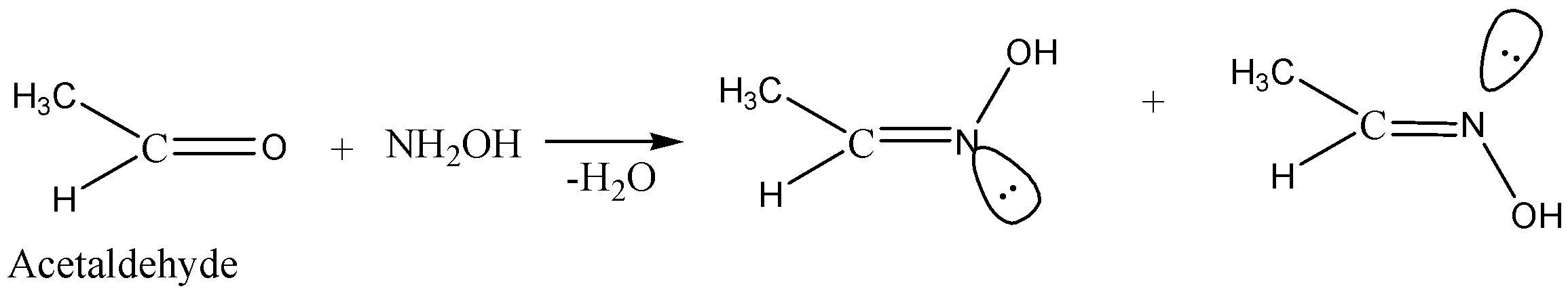 Hence the answer to this question is option A. $C{H_3}CHO$.
Hence the answer to this question is option A. $C{H_3}CHO$.
We have to remember that the formaldehyde does not form isomeric oxime because it is a symmetrical aldehyde and the ethyl methyl ketone and propyl ethyl ketone do not form oxime. Therefore, the options B, C and D are incorrect.
So, the correct answer is Option A.
Note: We must remember that the oximes are widely used in industry, oximes are used for metal extraction, Industrial production of caprolactam, used as antidotes for nerve agents etc. More than a million tonnes of cyclohexane is converted to oximes per year for meeting the production requirement of these materials.
Complete step by step answer:
Let’s start with discussing a little about oximes for better understanding of the question. Oximes is the class of nitrogen containing organic compounds which are generally prepared from hydroxylamine, aldehyde, ketones or Quinone. Oximes have a general molecular formula $RR^'$C=NO.
Where, R-alkyl group
R’-hydrogen
Coming back to the question we are asked for the molecule out of the options given to us which will form two oximes with $NH_2OH$. So, in this question we are provided with aldehydes and ketones as the option. Two isomeric oximes are possible with aldehydes (except formaldehydes) and non-symmetric ketones. Out of all the options given there is only one which fulfils the above condition and is capable of forming two isomeric oximes which is $C{H_3}CHO$.

We have to remember that the formaldehyde does not form isomeric oxime because it is a symmetrical aldehyde and the ethyl methyl ketone and propyl ethyl ketone do not form oxime. Therefore, the options B, C and D are incorrect.
So, the correct answer is Option A.
Note: We must remember that the oximes are widely used in industry, oximes are used for metal extraction, Industrial production of caprolactam, used as antidotes for nerve agents etc. More than a million tonnes of cyclohexane is converted to oximes per year for meeting the production requirement of these materials.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




