
Which two of the following aldohexoses give the same osazone derivative?
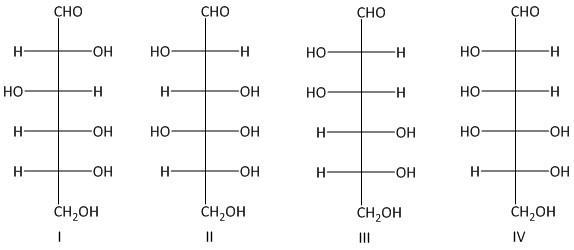
A. I and IV
B. I and III
C. II and III
D. III and IV

Answer
563.7k+ views
Hint:Aldohexoses is the common name of sugar molecules which contains an aldehyde group. The aldehyde or hydroxyl group reacts with phenylhydrazine to produce osazone compounds.
Complete step by step answer: The osazones are the derivatives of monosaccharides which was developed by Emil Fischer. The osazones of the carbohydrates are derived by treating the reducing sugars with excess of phenylhydrazine at boiling condition.
Osazones are organic compounds which imparts the structure of the corresponding sugar molecule. The product of the reaction leads to formation of a pair of phenylhydrazone functionalities. The probable mechanism involves the oxidation of the hydroxyl group at the alpha carbon with respect to the carbonyl group.
Thus the primary condition for osazone formation is that the C-1 carbon must have a carbonyl group and the C-2 carbon must have an oxidizable hydroxy group. The osazones obtained from two sugars which have identical alignment of hydrogen atoms and hydroxyl groups at C-3 , C-4 and C-5 carbon atoms are the same. So Glucosazone and fructosazone, for example, are identical.
Let us determine the corresponding osazones formed from the given set of aldohexoses:
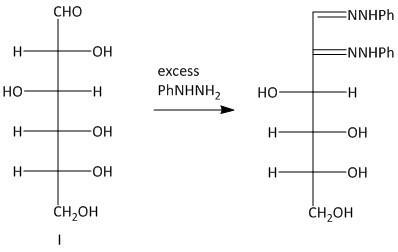
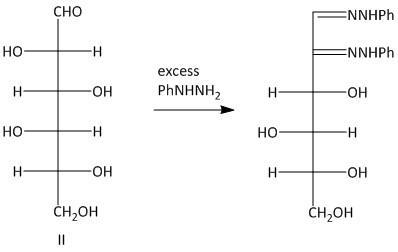
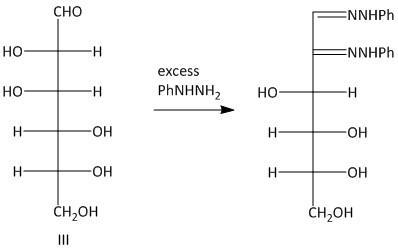
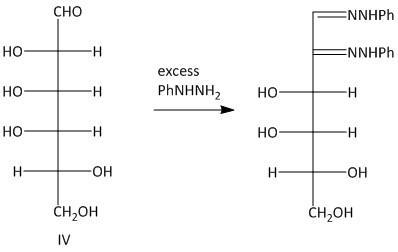
The osazones obtained from aldohexoses I and III are the same. Both of them contain a C-2 carbon epimer. Two water molecules liberated in the process of formation is osazone. The oxidation of the hydroxyl group at the alpha carbon to the aldehyde undergoes oxidation with a molecule of phenyl hydrazine. Thus a total of three molecules of phenyl hydrazine is required.
Hence option B is the correct answer.
Note:
The stereochemistry of the unknown chiral center of a monosaccharide is determined using an osazone test. The epimer is a pair of diastereomers which has an opposite configuration at one chiral center and all other centers have the same configuration.
Complete step by step answer: The osazones are the derivatives of monosaccharides which was developed by Emil Fischer. The osazones of the carbohydrates are derived by treating the reducing sugars with excess of phenylhydrazine at boiling condition.
Osazones are organic compounds which imparts the structure of the corresponding sugar molecule. The product of the reaction leads to formation of a pair of phenylhydrazone functionalities. The probable mechanism involves the oxidation of the hydroxyl group at the alpha carbon with respect to the carbonyl group.
Thus the primary condition for osazone formation is that the C-1 carbon must have a carbonyl group and the C-2 carbon must have an oxidizable hydroxy group. The osazones obtained from two sugars which have identical alignment of hydrogen atoms and hydroxyl groups at C-3 , C-4 and C-5 carbon atoms are the same. So Glucosazone and fructosazone, for example, are identical.
Let us determine the corresponding osazones formed from the given set of aldohexoses:




The osazones obtained from aldohexoses I and III are the same. Both of them contain a C-2 carbon epimer. Two water molecules liberated in the process of formation is osazone. The oxidation of the hydroxyl group at the alpha carbon to the aldehyde undergoes oxidation with a molecule of phenyl hydrazine. Thus a total of three molecules of phenyl hydrazine is required.
Hence option B is the correct answer.
Note:
The stereochemistry of the unknown chiral center of a monosaccharide is determined using an osazone test. The epimer is a pair of diastereomers which has an opposite configuration at one chiral center and all other centers have the same configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




