
Which statement is not correct for ortho and para hydrogen?
A) They have different boiling points.
B) Ortho-form is more stable than para-form.
C) They differ in their nuclear spin.
D) The ratio of ortho to para hydrogen changes with change in temperature.
Answer
573k+ views
Hint:The commonly known isomers of hydrogen based on its mass number are, protium, deuterium and tritium. Molecular hydrogen also has isomers based on its nuclear spin, namely ortho and para. The mixture of ortho and para forms of hydrogen is present in ordinary hydrogen molecules.
$[ortho{\text{ }}hydrogen \rightleftharpoons para{\text{ }}hydrogen] $
Complete answer:
Each hydrogen molecule is made up of two hydrogen atoms. Each hydrogen atom has one proton and one electron. In each hydrogen molecule, each the electrons spin around the nucleus and itself. Also, the nuclei of each of two hydrogen atoms in the molecule either spin in the same or different direction.
If in a hydrogen molecule, the nuclei of both hydrogen atoms are spinning in the same direction, then it is considered as ortho hydrogen.
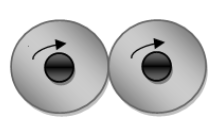
If in a hydrogen molecule, the nuclei of both hydrogen atoms are spinning in opposite directions, then it is considered as para hydrogen.
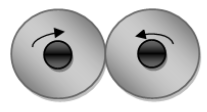
The two forms of hydrogen have different melting and boiling points and they have different vapour pressure.
The amount of ortho and para hydrogen present in a hydrogen molecule depends upon the temperature conditions. At room temperature, the ratio of ortho to para hydrogen in a hydrogen molecule is 3:1 that means a hydrogen molecule contains approximately 75% ortho and 25% para hydrogen. Even at high temperatures, the 3:1 ratio of ortho : para remain same. As the temperature starts decreasing, the amount of para hydrogen increases and that of ortho decreases. At 0°K, the hydrogen molecule contains mostly para hydrogen.
Therefore, at room temperature and higher temperature ortho form is dominant one whereas, at lower temperatures, para form is dominant one. Therefore, ortho form is stable than that of para form of hydrogen.
Hence, the correct option is option B
Note:
1. The two forms of hydrogen, ortho can be converted into para and vice versa. As ortho is more stable than para form, para can be converted into ortho by application of various methods.
2. The methods include reaction with catalysts like platinum or iron, passing of electric current through it, combining para hydrogen with nascent hydrogen and combining it with paramagnetic molecules.
$[ortho{\text{ }}hydrogen \rightleftharpoons para{\text{ }}hydrogen] $
Complete answer:
Each hydrogen molecule is made up of two hydrogen atoms. Each hydrogen atom has one proton and one electron. In each hydrogen molecule, each the electrons spin around the nucleus and itself. Also, the nuclei of each of two hydrogen atoms in the molecule either spin in the same or different direction.
If in a hydrogen molecule, the nuclei of both hydrogen atoms are spinning in the same direction, then it is considered as ortho hydrogen.

If in a hydrogen molecule, the nuclei of both hydrogen atoms are spinning in opposite directions, then it is considered as para hydrogen.

The two forms of hydrogen have different melting and boiling points and they have different vapour pressure.
The amount of ortho and para hydrogen present in a hydrogen molecule depends upon the temperature conditions. At room temperature, the ratio of ortho to para hydrogen in a hydrogen molecule is 3:1 that means a hydrogen molecule contains approximately 75% ortho and 25% para hydrogen. Even at high temperatures, the 3:1 ratio of ortho : para remain same. As the temperature starts decreasing, the amount of para hydrogen increases and that of ortho decreases. At 0°K, the hydrogen molecule contains mostly para hydrogen.
Therefore, at room temperature and higher temperature ortho form is dominant one whereas, at lower temperatures, para form is dominant one. Therefore, ortho form is stable than that of para form of hydrogen.
Hence, the correct option is option B
Note:
1. The two forms of hydrogen, ortho can be converted into para and vice versa. As ortho is more stable than para form, para can be converted into ortho by application of various methods.
2. The methods include reaction with catalysts like platinum or iron, passing of electric current through it, combining para hydrogen with nascent hydrogen and combining it with paramagnetic molecules.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




