
Which statement is incorrect for \[OS{F_4}\]?
A.S atom has \[s{p^3}d\] hybridization
B.\[OS{F_4}\]have distorted trigonal pyramidal geometry
C.O atom at one of the two axial positions having S=O bond
D.O atom at one of the two equatorial position having S=O bond
Answer
581.1k+ views
Hint: To solve this question, we must find the electronic configuration and then the hybridization. From this, we would be able to conclude the geometry of the molecule and then we can cross check the options given to us.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Let us first try to identify the hybridisation of the sulphur atom used and then in accordance to that, let us find the geometrical structure of the given compound.
Now, in the given compound, sulphur is the central atom. The atomic number of sulphur is 16. Hence, its electronic configuration can be given as: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}3{d^0}\]. Thus, we can see that there are 6 electrons present in the valence shell and these electrons are divided between the 3s and 3p subshells. The electrons in the valence shell can be represented as:
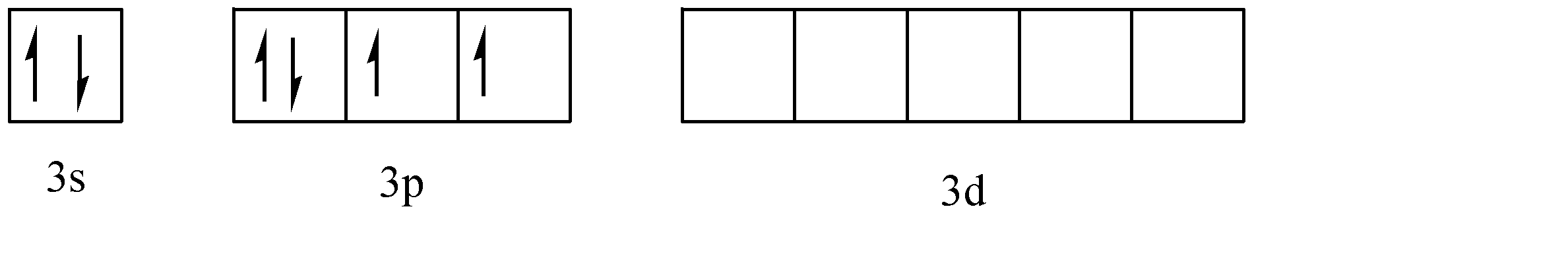
Now, we need to add 5 substituents to the sulphur atom to form \[OS{F_4}\], viz. 4 atoms of fluorine and one atom of oxygen. To form the sites for adding these substituents, the negative spinning electrons are transferred from their subshells to the ‘d’ subshell. Since 2 electrons are added to the ‘d’ orbital.
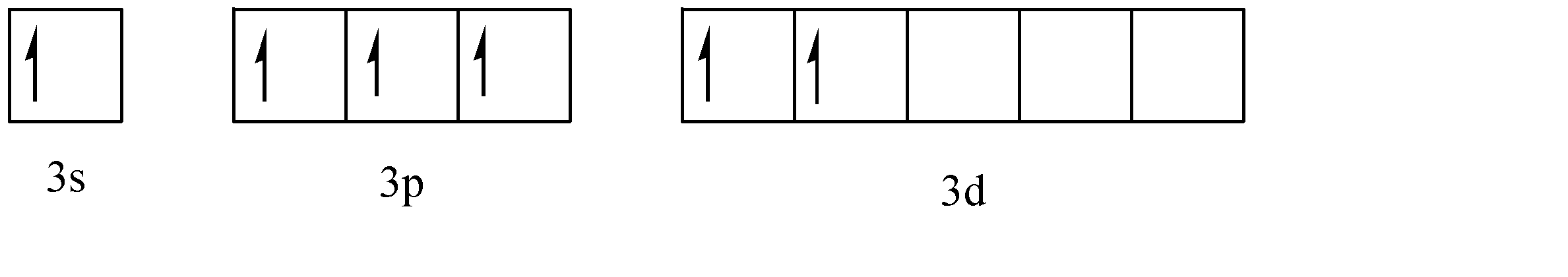
Now, one of the d orbital electrons will be required to form pi bonds with oxygen. Hence, the effective hybridization of the sulphur atom is \[s{p^3}d\]. Now, we have zero lone pairs, 5 sigma bonds and one pi bond present in the structure. Hence the corresponding geometric structure of \[OS{F_4}\] is trigonal bipyramidal. Hence the structure of \[OS{F_4}\] is given as:
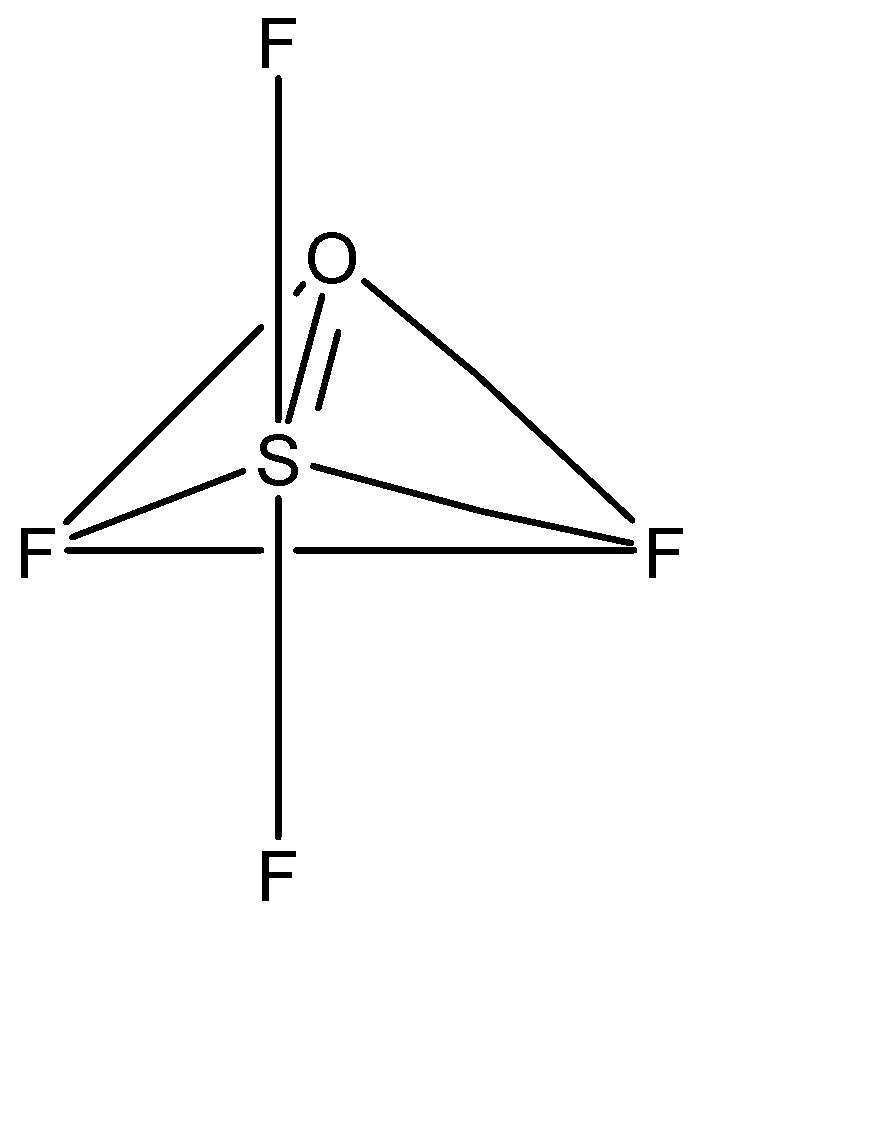
Hence, we can see that the oxygen is at the equatorial position.
Hence, Option C is the correct option
Note: Axial bonds can be understood as the bonds that are usually formed along the vertical axis of the central atom. On the other hand, equatorial bonds are formed at right angles to the axis of the central atom.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Let us first try to identify the hybridisation of the sulphur atom used and then in accordance to that, let us find the geometrical structure of the given compound.
Now, in the given compound, sulphur is the central atom. The atomic number of sulphur is 16. Hence, its electronic configuration can be given as: \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}3{d^0}\]. Thus, we can see that there are 6 electrons present in the valence shell and these electrons are divided between the 3s and 3p subshells. The electrons in the valence shell can be represented as:

Now, we need to add 5 substituents to the sulphur atom to form \[OS{F_4}\], viz. 4 atoms of fluorine and one atom of oxygen. To form the sites for adding these substituents, the negative spinning electrons are transferred from their subshells to the ‘d’ subshell. Since 2 electrons are added to the ‘d’ orbital.

Now, one of the d orbital electrons will be required to form pi bonds with oxygen. Hence, the effective hybridization of the sulphur atom is \[s{p^3}d\]. Now, we have zero lone pairs, 5 sigma bonds and one pi bond present in the structure. Hence the corresponding geometric structure of \[OS{F_4}\] is trigonal bipyramidal. Hence the structure of \[OS{F_4}\] is given as:

Hence, we can see that the oxygen is at the equatorial position.
Hence, Option C is the correct option
Note: Axial bonds can be understood as the bonds that are usually formed along the vertical axis of the central atom. On the other hand, equatorial bonds are formed at right angles to the axis of the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




