
Which Product is not possible in above reaction?
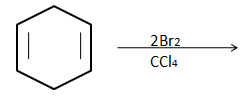
(A)
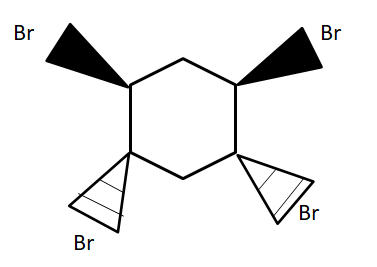
(B)
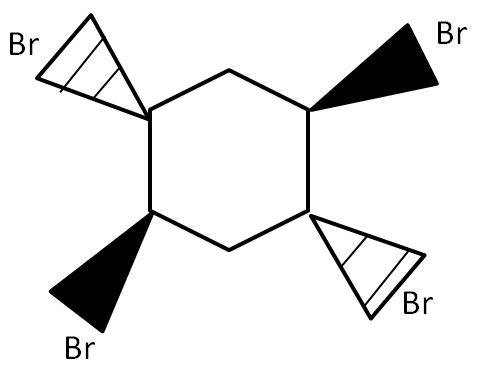
(C)
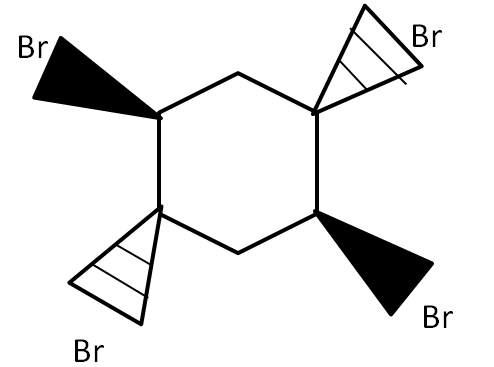
(D)
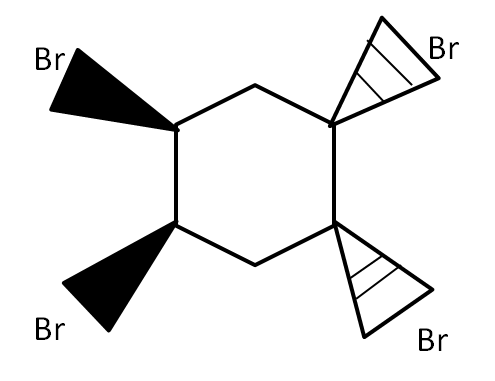
(E)
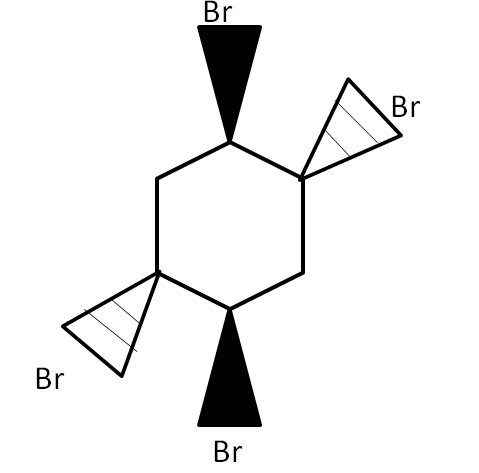






Answer
507.9k+ views
Hint :The aromatic compounds when after a reaction produces a compound connected to halogen then the reaction is termed as halogenations reaction. Iff in halogen we use a specific type of halogen then the reaction is named after that atom. For instance, iff bromine is used in halogenations, then it will be called a bromination reaction.
Complete Step By Step Answer:
The term aromatic is exclusively used when, the benzene or compounds resembling it is present in the complex. Thus if the benzene ring is present then the aromatic complexes would be termed as the benzenoid compounds like toluene, xylene, phenol, etc. Whereas iff benzene is not present, but a compound resembling to benzene like heme, pyridine are present then they would be regarded as the non-benzenoid compounds examples includes, azulene, ferrocene, etc.
For the bromination reactions, it is to remember that the addition of the bromine will always take place around the double bond carbons only. That means carbon single bond carbon is free from the reaction. However, for the product to form, it is very important to take account of the electron cloud. In all the options above only in option (A) there would be uneven distribution of cloud i.e., the bromine above the plane will take half of the cloud and below the plane will take another half of the cloud. This leads to the polarization of the ring which ultimately leads to the cleavage of the ring as it destabilizes. Any reaction which leads to instability of the compound will not take place with changing temperature, pressure and other conditions.
Thus the below structure is impossible to produce.
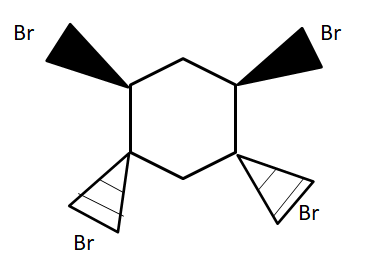
Note :
For the stable products the energy of the product should be lower than the reactants. And for a reaction to carry out without changing the temperature, pressure conditions, a reaction in which un-stable product is formed is not feasible. That is reaction will not take place.
Complete Step By Step Answer:
The term aromatic is exclusively used when, the benzene or compounds resembling it is present in the complex. Thus if the benzene ring is present then the aromatic complexes would be termed as the benzenoid compounds like toluene, xylene, phenol, etc. Whereas iff benzene is not present, but a compound resembling to benzene like heme, pyridine are present then they would be regarded as the non-benzenoid compounds examples includes, azulene, ferrocene, etc.
For the bromination reactions, it is to remember that the addition of the bromine will always take place around the double bond carbons only. That means carbon single bond carbon is free from the reaction. However, for the product to form, it is very important to take account of the electron cloud. In all the options above only in option (A) there would be uneven distribution of cloud i.e., the bromine above the plane will take half of the cloud and below the plane will take another half of the cloud. This leads to the polarization of the ring which ultimately leads to the cleavage of the ring as it destabilizes. Any reaction which leads to instability of the compound will not take place with changing temperature, pressure and other conditions.
Thus the below structure is impossible to produce.

Note :
For the stable products the energy of the product should be lower than the reactants. And for a reaction to carry out without changing the temperature, pressure conditions, a reaction in which un-stable product is formed is not feasible. That is reaction will not take place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




