
Which one of the following is a Lewis acid?
A) ${N_2}{H_4}$
B) \[R - OH\]
C) \[R - O - R\]
D) $AlC{l_3}$
Answer
578.1k+ views
Hint: To know which of the following is Lewis acid, try to remember the conditions of Lewis acid and Lewis base. Lewis acid- It receives the non- bonding electrons(electrophile). Lewis base- It contributes the non- bonding electron(nucleophile).
Complete answer:
Lewis theory provided the concept of Lewis acid and Lewis base. Since Lewis acid accepts a lone pair of electrons then it is an electron pair supporter or receiver. Various categories can act as Lewis acids. Lewis bases are electron pair donors.
Now, the conditions which determine Lewis acid are:
Simple positive charge ion such as: ${H^ + },F{e^{ + + }}$.
Substance whose central atom has incomplete octet such as: $AlC{l_3}$
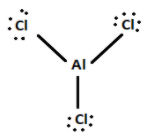
$AlC{l_3}$ is a Lewis acid. It is electron deficient. Al atoms have vacant orbitals and need two more electrons to complete its octet. It needs electrons from outside so it needs an electron pair from Cl. According to the definition, a substance which accepts a pair of non-bonding electrons is a Lewis acid. So $AlC{l_3}$ is a Lewis acid.
Thus, correct answer is option D.
Additional Information:
Conditions for Lewis base:
- Substance whose central atom has a lone pair of electrons such \[R - O - R\].
- Negative charge ions: \[{I^ - },C{l^ - }\].
Option A, ${N_2}{H_4}$ is known as Hydrazine.
In ${N_2}{H_4}$, each nitrogen has 1-1 lone pair. These lone pairs can be donated easily. So, it acts as a Lewis base.
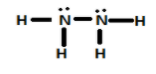
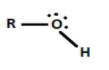
Option B, \[R - OH\] is a Lewis base because it has a lone pair (non-bonding) of electrons.
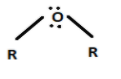
Option C, \[R - O - R\] is a Lewis base because the central atom has a lone pair of electrons.
Note: In each compound, try to memorize which reactant is electron deficient and which reactant is electron- pair donor. The advantage of Lewis theory is that it can expand a range of bases and acids.
Complete answer:
Lewis theory provided the concept of Lewis acid and Lewis base. Since Lewis acid accepts a lone pair of electrons then it is an electron pair supporter or receiver. Various categories can act as Lewis acids. Lewis bases are electron pair donors.
Now, the conditions which determine Lewis acid are:
Simple positive charge ion such as: ${H^ + },F{e^{ + + }}$.
Substance whose central atom has incomplete octet such as: $AlC{l_3}$

$AlC{l_3}$ is a Lewis acid. It is electron deficient. Al atoms have vacant orbitals and need two more electrons to complete its octet. It needs electrons from outside so it needs an electron pair from Cl. According to the definition, a substance which accepts a pair of non-bonding electrons is a Lewis acid. So $AlC{l_3}$ is a Lewis acid.
Thus, correct answer is option D.
Additional Information:
Conditions for Lewis base:
- Substance whose central atom has a lone pair of electrons such \[R - O - R\].
- Negative charge ions: \[{I^ - },C{l^ - }\].
Option A, ${N_2}{H_4}$ is known as Hydrazine.
In ${N_2}{H_4}$, each nitrogen has 1-1 lone pair. These lone pairs can be donated easily. So, it acts as a Lewis base.
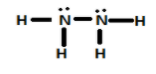
Option B, \[R - OH\] is a Lewis base because it has a lone pair (non-bonding) of electrons.

Option C, \[R - O - R\] is a Lewis base because the central atom has a lone pair of electrons.

Note: In each compound, try to memorize which reactant is electron deficient and which reactant is electron- pair donor. The advantage of Lewis theory is that it can expand a range of bases and acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




