
Which one of the following forms micelles in aqueous solution above certain concentration?
A. Urea
B. Dodecyl trimethyl ammonium chloride
C. Pyridinium chloride
D. Glucose
Answer
569.1k+ views
Hint: We can define micelles as supramolecular assembly of molecules of surfactant dispersed in liquid, and colloidal suspension is formed. A regular micelle in water is produced as an amassed hydrophilic head part in link with solvent impounding the hydrophobic single-tail regions in the center of micelle.
Complete step by step answer:
We need to know that the Micelles in aqueous solution above some concentrations are formed by detergents and soaps. We can say that an aggregate of surfactant molecules in a colloidal liquid is a micelle. Cationic detergents are those that give electrically positive ions in solution. The quaternary salts of ammonium of amines with acetate, chlorides or bromides as anions are cationic detergents. They contain cations at the soluble terminal of the chain. These are long-chain hydrocarbons containing a positive charge on nitrogen atoms. The long-chain cation accounts for their surface-active properties.
We must remember that the Dodecyl trimethyl ammonium chloride is a cationic detergent that forms micelles in the aqueous solution above particular concentration. We can draw the structure of dodecyl trimethyl ammonium chloride as,
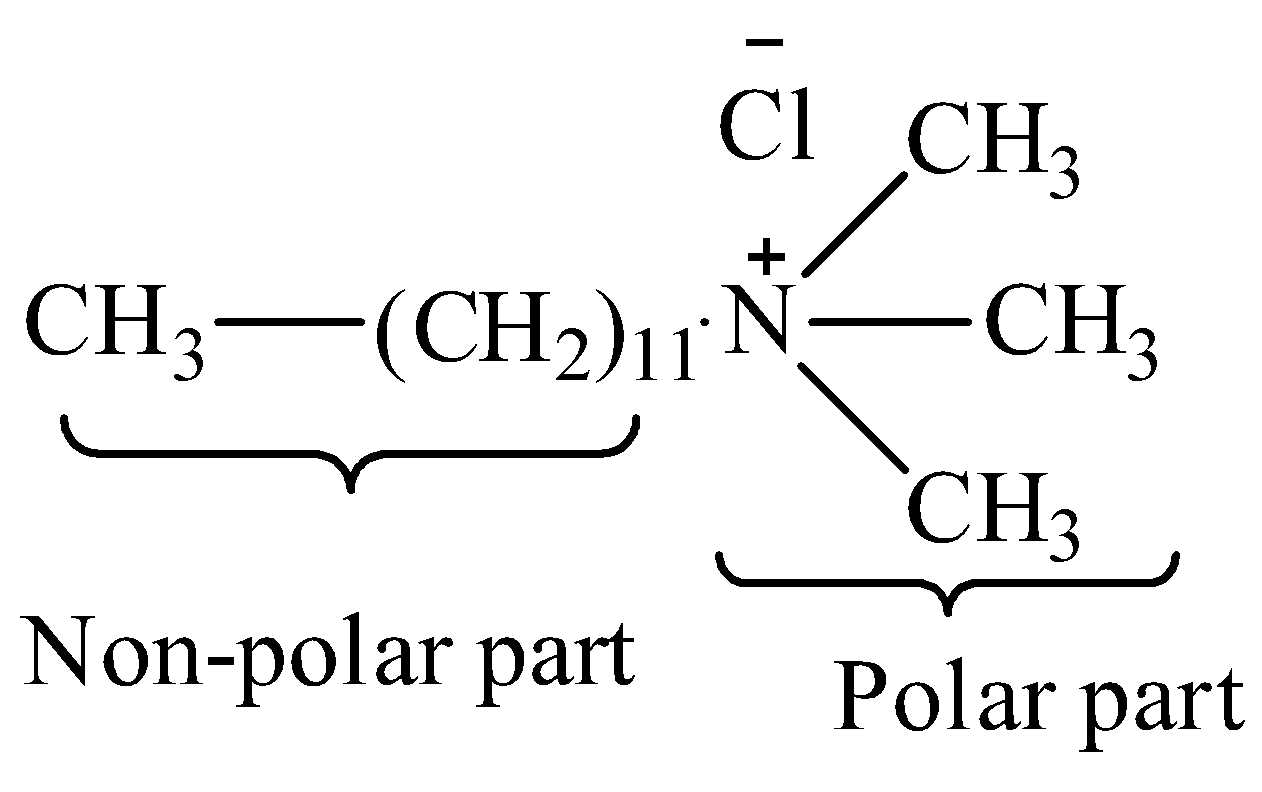
In aqueous media, in the presence of dodecyl trimethyl ammonium chloride and diclofenac sodium, micelles are formed because of their structure. Therefore, the option (B) is correct.
We know that urea, glucose, and pyridinium chloride are water-soluble as they are ionic compounds. Therefore, the options (A), (C), and (D) are incorrect.
So, the correct answer is Option B.
Note: We have to know that an example of another cationic surfactant that forms micelles in aqueous solution above a particular concentration is dodecyl dimethyl ethyl ammonium bromide. In fabric softeners and in detergents, cationic surfactants give softness. In laundry detergents, cationic surfactants enhance the packing of molecules of anionic surfactant at the interface of water/stain.
Complete step by step answer:
We need to know that the Micelles in aqueous solution above some concentrations are formed by detergents and soaps. We can say that an aggregate of surfactant molecules in a colloidal liquid is a micelle. Cationic detergents are those that give electrically positive ions in solution. The quaternary salts of ammonium of amines with acetate, chlorides or bromides as anions are cationic detergents. They contain cations at the soluble terminal of the chain. These are long-chain hydrocarbons containing a positive charge on nitrogen atoms. The long-chain cation accounts for their surface-active properties.
We must remember that the Dodecyl trimethyl ammonium chloride is a cationic detergent that forms micelles in the aqueous solution above particular concentration. We can draw the structure of dodecyl trimethyl ammonium chloride as,

In aqueous media, in the presence of dodecyl trimethyl ammonium chloride and diclofenac sodium, micelles are formed because of their structure. Therefore, the option (B) is correct.
We know that urea, glucose, and pyridinium chloride are water-soluble as they are ionic compounds. Therefore, the options (A), (C), and (D) are incorrect.
So, the correct answer is Option B.
Note: We have to know that an example of another cationic surfactant that forms micelles in aqueous solution above a particular concentration is dodecyl dimethyl ethyl ammonium bromide. In fabric softeners and in detergents, cationic surfactants give softness. In laundry detergents, cationic surfactants enhance the packing of molecules of anionic surfactant at the interface of water/stain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




