
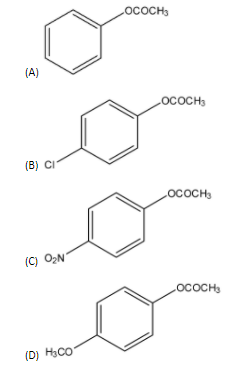
Which one of the following esters gets hydrolyzed most easily under alkaline conditions?

Answer
589.5k+ views
Hint: The substituents –H, -Cl, $ - N{O_2}$ and $ - OC{H_3}$ are neutral, electron donating, electron withdrawing and electron donating respectively. The compound which has the substituent that makes the carbonyl carbon most electrophilic will get hydrolyzed easily.
Complete step by step solution:
Let’s see the mechanism of the alkaline hydrolysis of esters in order to find which one of the given esters will undergo hydrolysis easily.
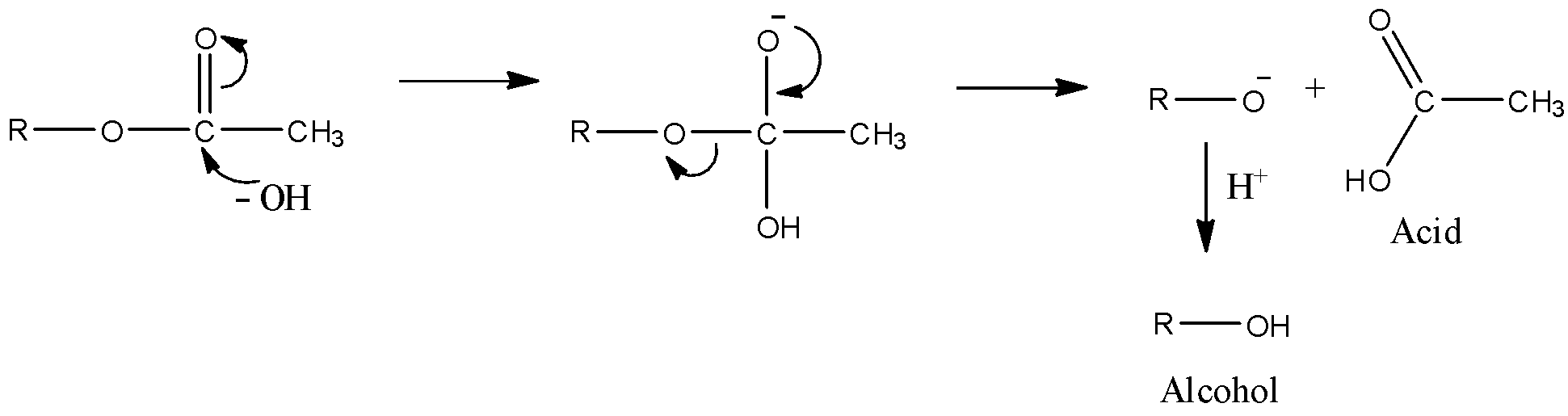
Here, we can see that the first step is the attack of the hydroxyl ion of the electrophilic carbon of the carbonyl group. Then, the alkoxy group leaves the molecule and they react with protons in order to form alcohol. An acid is also formed. Thus, upon hydrolysis of ester, alcohol and acid is formed.
- Now, in order to find which ester would get hydrolyzed easily, we need to see the first step. It will decide which acid will react more easily.
- We can say that if the carbonyl group is more electrophilic, then it would be more susceptible to the nucleophilic attack.
- We have four compounds in which substituents at para positions differ.
- Hydrogen atoms do not have any effect on the carbonyl carbon. Chlorine atoms at para position will increase the electron density on carbonyl carbon. So, it will also not facilitate the nucleophilic attack.
- Nitro group is a strong electron withdrawing group. So, it will make the carbonyl carbon most electrophilic. Thus, we obtain that this ester will be easier to hydrolyze. Methoxy group being an electron donating group will increase the electron density and thus, the electron density on carbon will increase.
Thus, we can say that compound in option (C) will be easily hydrolyzed. So, this is the correct answer.
Note: Note that chlorine and other halogen atoms can donate the electron density via resonance. However, they have high electronegativity, so they can accept electron density via inductive effect.
Complete step by step solution:
Let’s see the mechanism of the alkaline hydrolysis of esters in order to find which one of the given esters will undergo hydrolysis easily.

Here, we can see that the first step is the attack of the hydroxyl ion of the electrophilic carbon of the carbonyl group. Then, the alkoxy group leaves the molecule and they react with protons in order to form alcohol. An acid is also formed. Thus, upon hydrolysis of ester, alcohol and acid is formed.
- Now, in order to find which ester would get hydrolyzed easily, we need to see the first step. It will decide which acid will react more easily.
- We can say that if the carbonyl group is more electrophilic, then it would be more susceptible to the nucleophilic attack.
- We have four compounds in which substituents at para positions differ.
- Hydrogen atoms do not have any effect on the carbonyl carbon. Chlorine atoms at para position will increase the electron density on carbonyl carbon. So, it will also not facilitate the nucleophilic attack.
- Nitro group is a strong electron withdrawing group. So, it will make the carbonyl carbon most electrophilic. Thus, we obtain that this ester will be easier to hydrolyze. Methoxy group being an electron donating group will increase the electron density and thus, the electron density on carbon will increase.
Thus, we can say that compound in option (C) will be easily hydrolyzed. So, this is the correct answer.
Note: Note that chlorine and other halogen atoms can donate the electron density via resonance. However, they have high electronegativity, so they can accept electron density via inductive effect.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




