
Which of these molecules have non-bonding electron pairs on the central metal ion?
(I) $\text{S}{{\text{F}}_{\text{4}}}$ (II) $\text{IC}{{\text{l}}_{3}}$ (III) $\text{S}{{\text{O}}_{2}}$
(A) II only
(B) I and II only
(C) I and III only
(D) I, II and III
Answer
566.4k+ views
Hint: Non-bonding electron pair also known as lone pair. A nonbonding electron pair is an electron pair of valence electrons that are not involved in bonding with other atoms. It refers to a localized pair of electrons associated with an atom.
Complete Solution :
In a covalent molecule central atoms have binding and non-bonding pairs of electrons. Binding electrons are mainly involved in covalent bond formation, while non-binding electrons are present in the valence shell and or take part in co-ordinate bond formation.
(A) In \[\text{S}{{\text{F}}_{\text{4}}}\] sulphur atoms have six electrons in the valence shell. Out of six electrons four electrons participate in the binding with fluorine atoms. So the central atom contains two free electrons or a pair of nonbonding electrons, as shown in the following diagram.
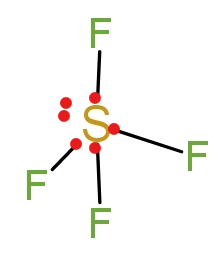
(B) In $\text{IC}{{\text{l}}_{\text{3}}}$, iodine atoms contain seven electrons in the valence shell. Out of seven electrons three electrons participate in the bonding with chlorine atoms. It contains four free electrons or two non-binding pairs of electrons which is represented in the following diagram where the dot represents the non- bonding electrons.

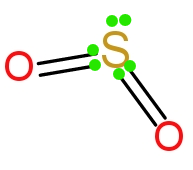
(C) In $\text{S}{{\text{O}}_{\text{2}}}$ sulphur atom contains six electrons in the valence shell. It is attached with two oxygen atoms through a double bond. Out of six electrons four electrons are involved in the bonding with oxygen atom. So the sulphur atom contains a pair of nonbonding electrons in the valence shell, which is represented in the following diagram.
Note: lone pair is the pair of valence electrons that are not involved in the bonding in a covalent molecule.
- A non-bonding pair of electrons present in the pure orbital of an atom if the electronegativity difference between two atoms is large. But a lone pair is present in the hybrid orbital if the electronegativity difference between central atom and bounded atom is low.
Complete Solution :
In a covalent molecule central atoms have binding and non-bonding pairs of electrons. Binding electrons are mainly involved in covalent bond formation, while non-binding electrons are present in the valence shell and or take part in co-ordinate bond formation.
(A) In \[\text{S}{{\text{F}}_{\text{4}}}\] sulphur atoms have six electrons in the valence shell. Out of six electrons four electrons participate in the binding with fluorine atoms. So the central atom contains two free electrons or a pair of nonbonding electrons, as shown in the following diagram.

(B) In $\text{IC}{{\text{l}}_{\text{3}}}$, iodine atoms contain seven electrons in the valence shell. Out of seven electrons three electrons participate in the bonding with chlorine atoms. It contains four free electrons or two non-binding pairs of electrons which is represented in the following diagram where the dot represents the non- bonding electrons.

(C) In $\text{S}{{\text{O}}_{\text{2}}}$ sulphur atom contains six electrons in the valence shell. It is attached with two oxygen atoms through a double bond. Out of six electrons four electrons are involved in the bonding with oxygen atom. So the sulphur atom contains a pair of nonbonding electrons in the valence shell, which is represented in the following diagram.

Note: lone pair is the pair of valence electrons that are not involved in the bonding in a covalent molecule.
- A non-bonding pair of electrons present in the pure orbital of an atom if the electronegativity difference between two atoms is large. But a lone pair is present in the hybrid orbital if the electronegativity difference between central atom and bounded atom is low.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




