
Which of the statements is correct about \[{\text{S}}{{\text{O}}_2}\] ?
(A) It contains two sigma, two pi bonds and no lone pair of electrons.
(B) It contains two sigma and one pi bond.
(C) It contains two sigma, two pi bonds and one lone pair of electrons.
(D) None of the above.
Answer
579.6k+ views
Hint: Obtain the number of valence electrons of the central sulphur atom and each oxygen atom. Form bonds so that the octet of each oxygen atom is completed.
Complete answer:
The molecule \[{\text{S}}{{\text{O}}_2}\] is a sulphur dioxide molecule. In this molecule, the central atom is the sulphur atom. The number of valence electrons present in a sulphur atom is 6. Similarly, the number of valence electrons present in an oxygen atom is also 6. Out of 6 valence electrons of sulphur atom, two are shared with two oxygen atoms to form two sulphur-oxygen sigma bonds. Also shared are two valence electrons of sulphur atoms with two oxygen atoms to form two sulphur-oxygen pi bonds. Thus, out of six valence electrons of sulphur, four are used up and only two are remaining. These two-valence electrons are present as one lone pair of electrons.
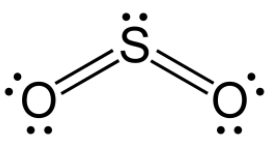
Thus, sulphur dioxide molecules contain two sigma, two pi bonds and one lone pair of electrons.
Hence, the correct option is the option (C).
Additional Information: The central sulphur atom in sulphur dioxide molecule has two bonding domains and a lone pair of electrons. The steric number of sulphur is 3. The steric number of 3 is associated with \[{\text{s}}{{\text{p}}^2}\] hybridization. The electron pair geometry is trigonal planar and the molecular geometry is bend or V shaped.
Note: According to octet rule, an atom either loses, or gains or shares electrons so that its valence shell contains eight electrons. This is in order to attain stability. Hydrogen is an exception as it needs only 2 electrons in its valence electron. Some atoms such as phosphorus or sulphur form compounds with expanded octet in which they have more than 8 valence electrons. Similarly, some atoms form incomplete octets with less than 8 valence electrons.
Complete answer:
The molecule \[{\text{S}}{{\text{O}}_2}\] is a sulphur dioxide molecule. In this molecule, the central atom is the sulphur atom. The number of valence electrons present in a sulphur atom is 6. Similarly, the number of valence electrons present in an oxygen atom is also 6. Out of 6 valence electrons of sulphur atom, two are shared with two oxygen atoms to form two sulphur-oxygen sigma bonds. Also shared are two valence electrons of sulphur atoms with two oxygen atoms to form two sulphur-oxygen pi bonds. Thus, out of six valence electrons of sulphur, four are used up and only two are remaining. These two-valence electrons are present as one lone pair of electrons.

Thus, sulphur dioxide molecules contain two sigma, two pi bonds and one lone pair of electrons.
Hence, the correct option is the option (C).
Additional Information: The central sulphur atom in sulphur dioxide molecule has two bonding domains and a lone pair of electrons. The steric number of sulphur is 3. The steric number of 3 is associated with \[{\text{s}}{{\text{p}}^2}\] hybridization. The electron pair geometry is trigonal planar and the molecular geometry is bend or V shaped.
Note: According to octet rule, an atom either loses, or gains or shares electrons so that its valence shell contains eight electrons. This is in order to attain stability. Hydrogen is an exception as it needs only 2 electrons in its valence electron. Some atoms such as phosphorus or sulphur form compounds with expanded octet in which they have more than 8 valence electrons. Similarly, some atoms form incomplete octets with less than 8 valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




