
Which of the following will not be able to show optical isomerism (enantiomerism)?
A. I,2-Propadiene
B. 2,3-Pentadiene
C. Sec-Butyl alcohol
D. All exhibit enantiomerism
Answer
537k+ views
Hint: Now the molecule, which is achiral, cannot show optical isomerism (enantiomerism) and achiral molecule is a molecule which is superimposed on its mirror image. Draw the structure of all the four options given above and see whichever molecule is achiral will not show optical isomerism (enantiomerism).
Complete step by step solution:
In chemistry there is a basic term isomerism. Isomerism occurs when certain compounds, having seen molecular formulas differ in the arrangement of their structure. There are different kinds of isomerism; one such isomerism is known as stereoisomerism. In stereoisomerism, compounds have the same molecular formula and same structure formula. But they differ in the arrangement of their 3D structure that is rotation of the bond.
Now, stereoisomerism contains another term that is called optical isomerism. In this, compounds having the same molecular formula have the same structure formula, but they only differ when a polarized light fall on them. The direction in which the polarized light rotates is left or right, that defers the compound.
Now we will draw the structure of the three options given above to see which of them generates a optical isomer (enantiomerism)
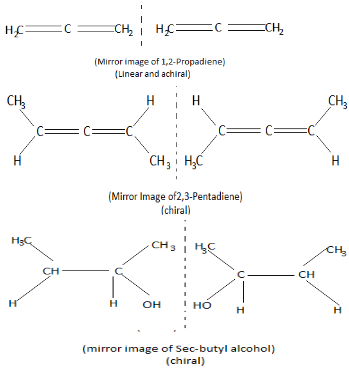
Thus the correct answer to the given question is option A.
Note: Remember that if the structure of a molecule is linear it is always achiral, that is, it's always super imposable on its mirror image. Molecules which have non-linear structure can be a chiral molecule. Also, achiral molecules do not create optical isomers.
Complete step by step solution:
In chemistry there is a basic term isomerism. Isomerism occurs when certain compounds, having seen molecular formulas differ in the arrangement of their structure. There are different kinds of isomerism; one such isomerism is known as stereoisomerism. In stereoisomerism, compounds have the same molecular formula and same structure formula. But they differ in the arrangement of their 3D structure that is rotation of the bond.
Now, stereoisomerism contains another term that is called optical isomerism. In this, compounds having the same molecular formula have the same structure formula, but they only differ when a polarized light fall on them. The direction in which the polarized light rotates is left or right, that defers the compound.
Now we will draw the structure of the three options given above to see which of them generates a optical isomer (enantiomerism)

Thus the correct answer to the given question is option A.
Note: Remember that if the structure of a molecule is linear it is always achiral, that is, it's always super imposable on its mirror image. Molecules which have non-linear structure can be a chiral molecule. Also, achiral molecules do not create optical isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




