
Which of the following will most readily give the hydrogenation product?
A.
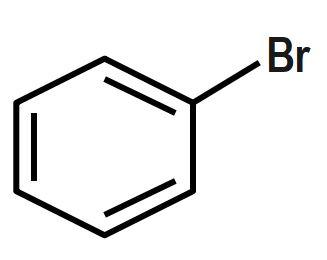
B.
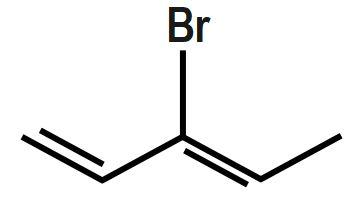
C.
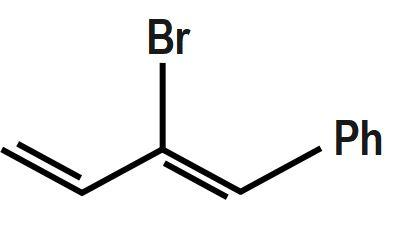
D.
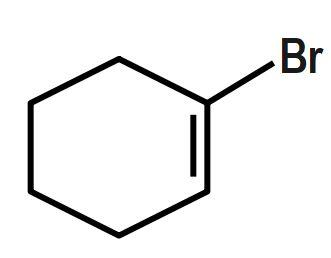




Answer
523.2k+ views
Hint: We know that the rate of formation of alkene will depend on the stability of alkene. More stable alkene will form rapidly and less stable alkene will take form slowly. The stability of alkene is determined through hyperconjugation.
Complete answer:
When the hydration of alcohol occurs under acidic condition alkene is formed. The ease of dehydration depends upon the stability of the alkene formed. Greater is the stability of alkene, easier it would be to form that alkene. The stability of alkene depends upon the hyperconjugation. More is the hyperconjugation which will be the stability of alkene. Hyperconjugation depends upon the number of Alpha hydrogens, higher is the number of Alpha hydrogen, more will be hyperconjugation. So we need to check the number of Alpha hydrogen that is present in the alkene that is formed by the given compounds.
It has two carbons next to the double bond. There is no hydrogen on carbon that is on RHS but there are three hydrogen atoms on LHS. So this molecule has three alpha hydrogen. Here dehydrohalogenation goes by\[~E1cB~\] and most stable carbanion formation is favored in A.
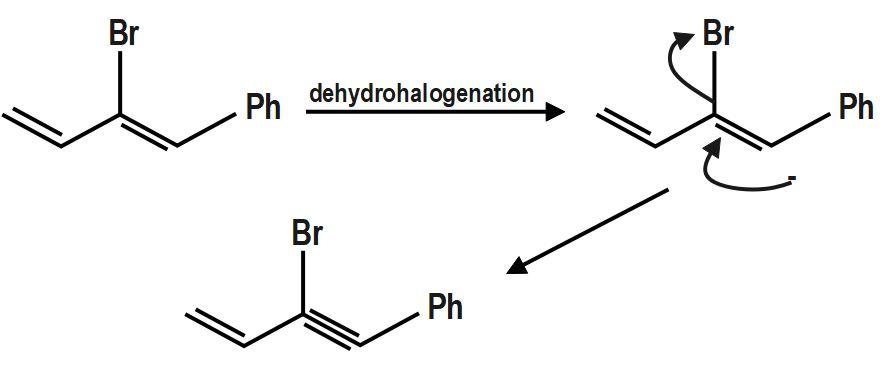
Therefore, the correct answer is option C.
Note:
Remember that if the question is asked with multiple answers then we will mark both option A and D correct. If there is only one option to choose from then the option D is marked as correct as the stability of the carbocation formed in option D is higher than the stability of the carbocation formed in option A.
Complete answer:
When the hydration of alcohol occurs under acidic condition alkene is formed. The ease of dehydration depends upon the stability of the alkene formed. Greater is the stability of alkene, easier it would be to form that alkene. The stability of alkene depends upon the hyperconjugation. More is the hyperconjugation which will be the stability of alkene. Hyperconjugation depends upon the number of Alpha hydrogens, higher is the number of Alpha hydrogen, more will be hyperconjugation. So we need to check the number of Alpha hydrogen that is present in the alkene that is formed by the given compounds.
It has two carbons next to the double bond. There is no hydrogen on carbon that is on RHS but there are three hydrogen atoms on LHS. So this molecule has three alpha hydrogen. Here dehydrohalogenation goes by\[~E1cB~\] and most stable carbanion formation is favored in A.

Therefore, the correct answer is option C.
Note:
Remember that if the question is asked with multiple answers then we will mark both option A and D correct. If there is only one option to choose from then the option D is marked as correct as the stability of the carbocation formed in option D is higher than the stability of the carbocation formed in option A.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




