
Which of the following statement(s) is/are correct about the tropolone?
A. Tropolone has more stability and aromatic character than tropane.
B. Tropolone has a higher dipole moment than tropane.
C. Tropolone has a lower boiling point than tropane.
D. All are correct.
Answer
579.9k+ views
Hint: For a compound to be considered too aromatic in nature, it must obey Huckel's Rule. Huckle’s rule states that the number of electrons present in a compound should be equal to \[\left( {4n + 2} \right)\pi \] , where n represents any natural number and is not limited to any specific set of values. Now, \[\pi \] electrons can be understood as the electrons that are present in the \[\pi \] bonds of a double bond or a triple bond or in a conjugated p – orbital.
Complete step by step answer:
Tropolone has more stability and aromatic character than tropane. This is because the lone pair of the hydroxyl group undergoes resonance, therefore the resonance stability increases. But this kind of stability is not observed in tropane.
The resonance with the hydroxyl group makes the dipole moment of tropolone less than tropane.
In the case of a tropolone, it can form intermolecular hydrogen bonding with its hydroxyl groups, but on the other hand, tropane cannot form hydrogen bonding. Due to this reason, Tropolone has a lower boiling point than tropane.
The structures of tropane and tropolone are shown below,
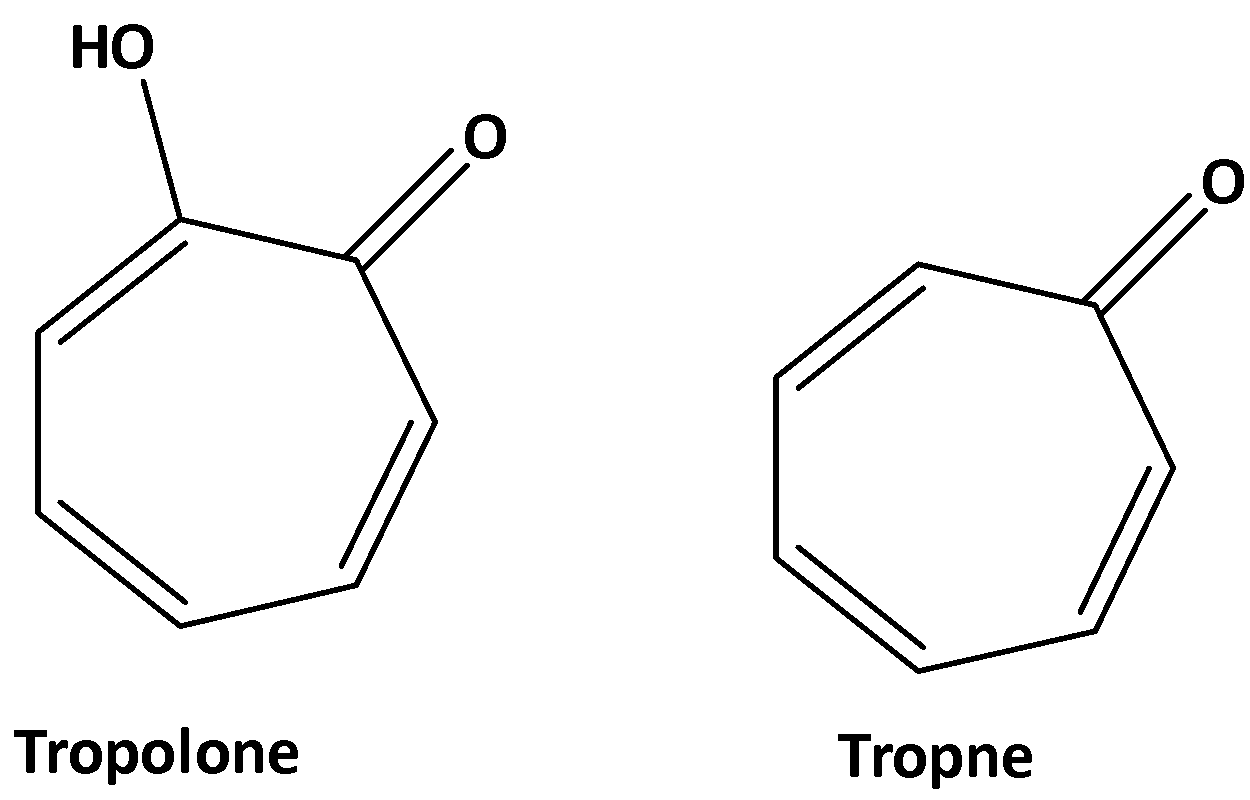
So, the correct option is D.
Note: Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature.
Complete step by step answer:
Tropolone has more stability and aromatic character than tropane. This is because the lone pair of the hydroxyl group undergoes resonance, therefore the resonance stability increases. But this kind of stability is not observed in tropane.
The resonance with the hydroxyl group makes the dipole moment of tropolone less than tropane.
In the case of a tropolone, it can form intermolecular hydrogen bonding with its hydroxyl groups, but on the other hand, tropane cannot form hydrogen bonding. Due to this reason, Tropolone has a lower boiling point than tropane.
The structures of tropane and tropolone are shown below,

So, the correct option is D.
Note: Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




