
Which of the following statements is not true about canal rays?
A) They travel in straight line
B) They are made of positively charged particles
C) Their e/m ratio is same for all gases
D) These rays produce fluorescence on the ZnS screen.
Answer
578.4k+ views
Hint: Refer to the Goldstein experiment of canal rays. Goldstein was the early investigator of discharge tubes and also the discoverer of the canal rays, also called anode rays. Recall the results of his experiment.
Complete answer:
Canal ray experiment performed by the German scientist Eugen Goldstein in 1886. This experiment led to the discovery of protons. This discovery happened just after the discovery of electrons. In the experiment, Goldstein applied high voltage across a discharge tube which had a perforated cathode. When the voltage was increased to several thousand volts, a faint luminous ray or a faint red glow was observed from the holes behind the cathode on a zinc sulphide screen. These rays were named canal rays, also called anode rays (carrying positive charge particles). The experiment is depicted below:
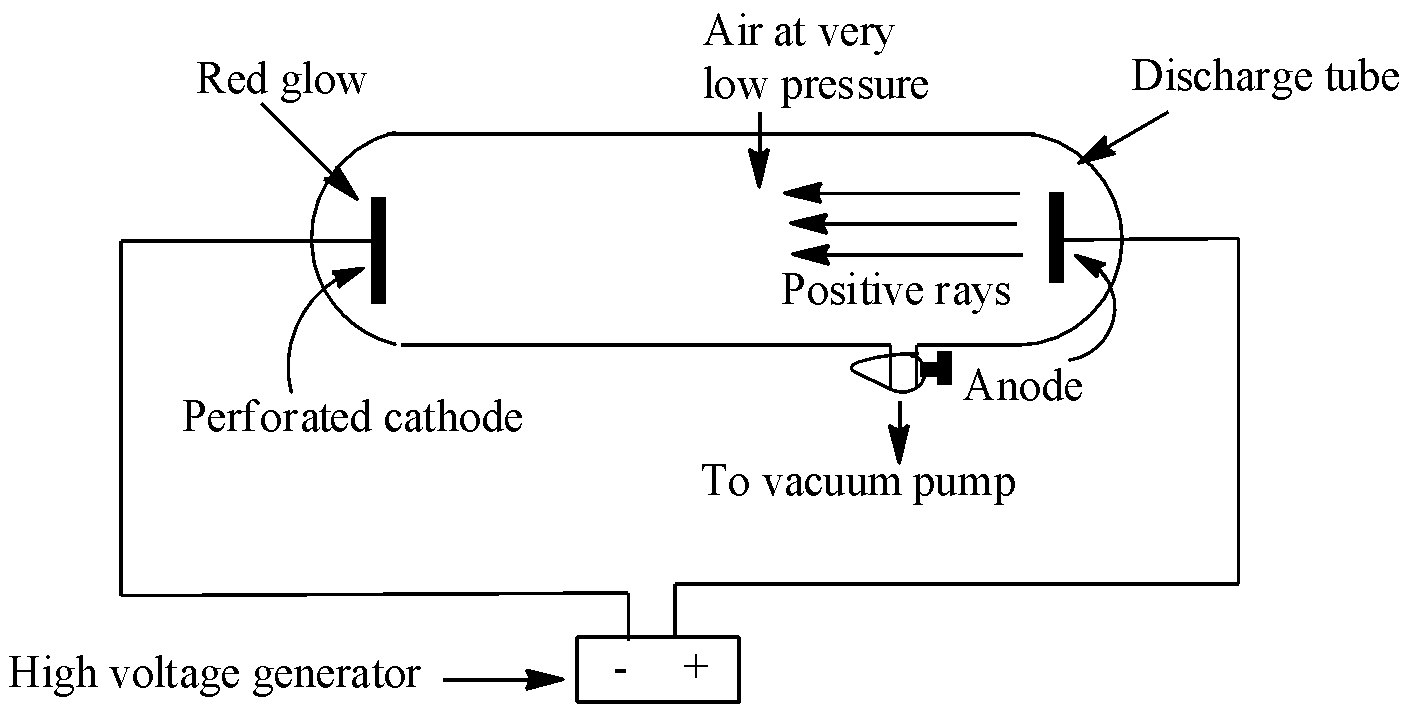
The characteristic of these canal rays are listed below:
- Canal rays are made up of positively charged particles
- In the absence of electrical and magnetic fields, these rays travel in a straight line from anode to cathode.
- The charge to mass ratio or e/m ratio of the canal rays is found to depend on the nature of gas i.e, not same for all gases.
- They produce fluorescence on the ZnS screen.
Hence, among all the given options, the statement given in option C is not the characteristic of canal rays.
Thus, option C is the answer.
Note:
Goldstein also took his own investigations of discharge tubes and cathode rays. He discovered several important properties of cathode rays, which later contributed to the discovery of electrons. Cathode rays travel from negatively charged cathode towards the positively charged anode while anode rays travel in the opposite direction.
Complete answer:
Canal ray experiment performed by the German scientist Eugen Goldstein in 1886. This experiment led to the discovery of protons. This discovery happened just after the discovery of electrons. In the experiment, Goldstein applied high voltage across a discharge tube which had a perforated cathode. When the voltage was increased to several thousand volts, a faint luminous ray or a faint red glow was observed from the holes behind the cathode on a zinc sulphide screen. These rays were named canal rays, also called anode rays (carrying positive charge particles). The experiment is depicted below:

The characteristic of these canal rays are listed below:
- Canal rays are made up of positively charged particles
- In the absence of electrical and magnetic fields, these rays travel in a straight line from anode to cathode.
- The charge to mass ratio or e/m ratio of the canal rays is found to depend on the nature of gas i.e, not same for all gases.
- They produce fluorescence on the ZnS screen.
Hence, among all the given options, the statement given in option C is not the characteristic of canal rays.
Thus, option C is the answer.
Note:
Goldstein also took his own investigations of discharge tubes and cathode rays. He discovered several important properties of cathode rays, which later contributed to the discovery of electrons. Cathode rays travel from negatively charged cathode towards the positively charged anode while anode rays travel in the opposite direction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




