
Which of the following statements is correct about the given Daniel cell?
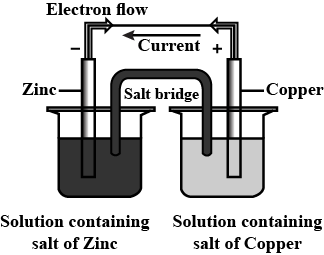
(A) This cell converts the electrical energy liberated during the redox reaction to chemical energy.
(B) This cell has an electrical potential greater than 1.1V when concentration of $Z{{n}^{2+}}$ and $C{{u}^{2+}}$ ions by unity $(1mol/d{{m}^{3}})$
(C) In this cell, zinc is acting as anode and copper is acting as cathode.
(D) Redox reaction occurring in this cell is $Cu(s)+Z{{n}^{2+}}(aq)\to C{{u}^{2+}}(aq)+Zn(s)$

Answer
569.7k+ views
Hint: Revise the concepts of electrochemical cells. A Daniel cell is a galvanic cell, so it converts chemical energy into electrical energy. Think about the electrochemical series and accordingly write the half-cell reactions taking place at cathode and anode and then determine the most suitable option for answer.
Complete Solution :
We have come across the concepts of various cells used for the measurement of concentration, pH and many other parameters.
We shall now see the basic Daniel cell and deduce the answer accordingly from the diagram of Daniel cell given in the question.
- Daniel cell is a galvanic cell and in this chemical energy is converted into electrical energy.
- According to the electrochemical series of reactivity of metals, zinc has more tendency to undergo oxidation than copper.
- So, the half-cell reactions according to the given diagram in data will be:
\[Zn\to Z{{n}^{2+}}+2{{e}^{-}}\] (at anode - oxidation)
\[C{{u}^{2+}}+2{{e}^{-}}\to Cu\] (at cathode - reduction)
- Therefore, the overall reaction is given as,
\[Zn+C{{u}^{2+}}\to Z{{n}^{2+}}+Cu\]
- This is the redox reaction which is taking place in Daniel's cell.
- The potential of the cell is 1.1 V initially.
- Therefore, the only correct statement given in the options is zinc is the anode and copper forms the cathode.
Note: Remember in galvanic cells, according to the electrochemical series, the metal which undergoes oxidation is anode and the metal undergoing reduction is cathode. In a galvanic cell, oxidation always takes place at anode and anode will have negative charge. At cathode, reduction will take place, and therefore, cathode will have positive charge.
Complete Solution :
We have come across the concepts of various cells used for the measurement of concentration, pH and many other parameters.
We shall now see the basic Daniel cell and deduce the answer accordingly from the diagram of Daniel cell given in the question.
- Daniel cell is a galvanic cell and in this chemical energy is converted into electrical energy.
- According to the electrochemical series of reactivity of metals, zinc has more tendency to undergo oxidation than copper.
- So, the half-cell reactions according to the given diagram in data will be:
\[Zn\to Z{{n}^{2+}}+2{{e}^{-}}\] (at anode - oxidation)
\[C{{u}^{2+}}+2{{e}^{-}}\to Cu\] (at cathode - reduction)
- Therefore, the overall reaction is given as,
\[Zn+C{{u}^{2+}}\to Z{{n}^{2+}}+Cu\]
- This is the redox reaction which is taking place in Daniel's cell.
- The potential of the cell is 1.1 V initially.
- Therefore, the only correct statement given in the options is zinc is the anode and copper forms the cathode.
Note: Remember in galvanic cells, according to the electrochemical series, the metal which undergoes oxidation is anode and the metal undergoing reduction is cathode. In a galvanic cell, oxidation always takes place at anode and anode will have negative charge. At cathode, reduction will take place, and therefore, cathode will have positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




