
Which of the following shoes are tautomerize?
(A)
(B)
(C)
(D)




Answer
509.7k+ views
Hint :the phenomena in which different compounds having the same chemical formula but different structures are known as isomerism and the compounds exhibiting isomerism are known as isomers. Isomerism is an important phenomenon for compounds.
Complete Step By Step Answer:
Isomerism as explained earlier are of different types. Broadly they are divided into structural isomers and stereoisomers. Structural isomers are further divided into chain isomers, position isomers and functional isomers. Whereas stereoisomers are of two types namely geometric and optical isomers.
Remember that the functional group isomers have different functional groups. For example $ Acetone $ and $ propanal $ are functional isomers as they contain different functional groups.
Tautomers are also functional isomers. Tautomerism is a phenomenon where a single chemical compound tends to exist in two or more interconvertible structures that are different in terms of relative position of one atomic nucleus which is generally the hydrogen. Structures which exhibit tautomerism are called tautomers and these types of isomers compounds usually differ only in number of electrons and protons.
Tautomerism generally occurs in planar or no planar molecules and has no effect on bond length . It involves the change of position of an atom. For tautomerism, the structural requirement of a compound is that it should contain a weakly acidic functional group and a polar molecule.
Most common type of tautomerism is keto-enol tautomerism. This arises due to $ 1,3 - $ migration of the hydrogen atom from carbon to oxygen atom. Also keep in mind that if a compound has an alpha-hydrogen atom, only then the compound can show keto-enol tautomerism.
Let's explore the options provided to us.
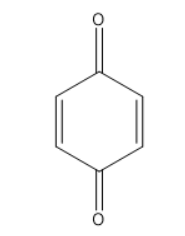
In option a, the structure is benzoquinone. If you remember the two conditions for keto-enol tautomerism, it doesn’t have alpha-hydrogen. Therefore it does not show tautomerism.
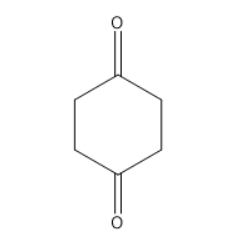
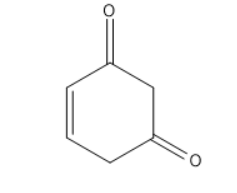
Similarly for the second and third option they also do not contain alpha-hydrogen.
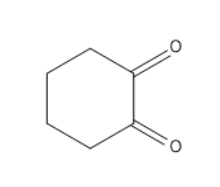
Now coming to the option d, which is $ 1,2 - Cyclohexanedione $ has an alpha carbon therefore it will show tautomerism.
Therefore our answer is D.
Note :
Alpha-hydrogen is a hydrogen atom on an alpha carbon in an organic molecule. Alpha carbon refers to the first carbon that attaches to the functional group, such as carbonyl. The hydrogen attached to this carbon is known as alpha hydrogen. Adjacent to alpha carbon are beta carbon and so on.
Complete Step By Step Answer:
Isomerism as explained earlier are of different types. Broadly they are divided into structural isomers and stereoisomers. Structural isomers are further divided into chain isomers, position isomers and functional isomers. Whereas stereoisomers are of two types namely geometric and optical isomers.
Remember that the functional group isomers have different functional groups. For example $ Acetone $ and $ propanal $ are functional isomers as they contain different functional groups.
Tautomers are also functional isomers. Tautomerism is a phenomenon where a single chemical compound tends to exist in two or more interconvertible structures that are different in terms of relative position of one atomic nucleus which is generally the hydrogen. Structures which exhibit tautomerism are called tautomers and these types of isomers compounds usually differ only in number of electrons and protons.
Tautomerism generally occurs in planar or no planar molecules and has no effect on bond length . It involves the change of position of an atom. For tautomerism, the structural requirement of a compound is that it should contain a weakly acidic functional group and a polar molecule.
Most common type of tautomerism is keto-enol tautomerism. This arises due to $ 1,3 - $ migration of the hydrogen atom from carbon to oxygen atom. Also keep in mind that if a compound has an alpha-hydrogen atom, only then the compound can show keto-enol tautomerism.
Let's explore the options provided to us.
In option a, the structure is benzoquinone. If you remember the two conditions for keto-enol tautomerism, it doesn’t have alpha-hydrogen. Therefore it does not show tautomerism.
Similarly for the second and third option they also do not contain alpha-hydrogen.
Now coming to the option d, which is $ 1,2 - Cyclohexanedione $ has an alpha carbon therefore it will show tautomerism.
Therefore our answer is D.
Note :
Alpha-hydrogen is a hydrogen atom on an alpha carbon in an organic molecule. Alpha carbon refers to the first carbon that attaches to the functional group, such as carbonyl. The hydrogen attached to this carbon is known as alpha hydrogen. Adjacent to alpha carbon are beta carbon and so on.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




