
Which of the following reagents is not suitable for the elimination reaction $?$
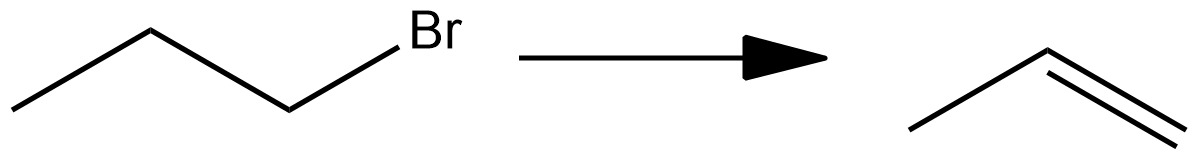
$(1)$ NaI
$(2)$ NaOEt $|$ EtOH
$(3)$ NaOH $|$ ${H_2}O$
$(4)$ NaOH $|$ ${H_2}O$ - EtOH

Answer
514.2k+ views
Hint : The given reaction shows an elimination reaction of bromopropane to propene. The bromine atom along with the hydrogen atom of the $\beta $ carbon gets eliminated as HBr to form a double bond. Now, we have to find which among the given reagents is not suitable for the elimination reaction to occur. Strong nucleophiles will give an elimination reaction rather than a substitution reaction.
Complete Step By Step Answer:
Elimination reactions are endothermic organic reactions that are used to prepare alkenes from alkyl halides and alcohols.
Strong nucleophiles act as better reagents for elimination reactions. Now we have to find the strong nucleophiles among the given ones. $\mathop O\limits^ - \,Et$ and $O{H^ - }$ are strong bases and strong nucleophiles and in the presence of strong bases like ethanol, they promote elimination reaction. The base abstracts $\beta $ hydrogen from the alkyl halide.
Iodide ion, ${I^ - }$, is a weak base and therefore a weak nucleophile. Therefore, if we use NaI, a substitution reaction takes place ($S{N_2}$ ). In $S{N_2}$ mechanism, the incoming nucleophile (${I^ - }$ ) approaches in a direction opposite to the leaving group ( $B{r^ - }$ ) and a bond is made and broken simultaneously involving a five-membered transition state. This mechanism occurs in substitution reactions in primary alkyl halides. The bromide in bromopropane will be replaced by iodide ion. We get iodopropane as a product instead of an alkene. It does not favour elimination reactions. The reaction is as follows:
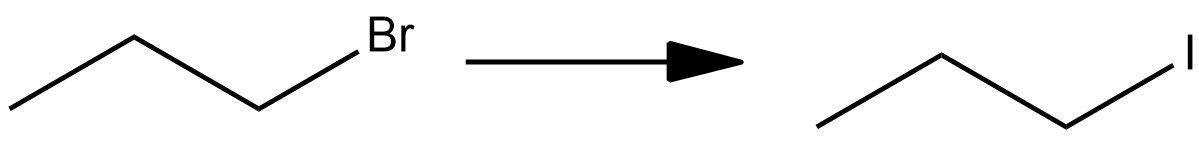
The right option is $(1)$ NaI.
Note :
The carbon atom to which the functional group is added, is called as $\alpha $ carbon and the immediate neighbouring carbon atom attached to $\alpha $ carbon is known as $\beta $ carbon. Hydrogen atoms attached to $\alpha $ carbon are called $\alpha $ hydrogen, and those attached to $\beta $ carbon are called $\beta $ hydrogen.
Complete Step By Step Answer:
Elimination reactions are endothermic organic reactions that are used to prepare alkenes from alkyl halides and alcohols.
Strong nucleophiles act as better reagents for elimination reactions. Now we have to find the strong nucleophiles among the given ones. $\mathop O\limits^ - \,Et$ and $O{H^ - }$ are strong bases and strong nucleophiles and in the presence of strong bases like ethanol, they promote elimination reaction. The base abstracts $\beta $ hydrogen from the alkyl halide.
Iodide ion, ${I^ - }$, is a weak base and therefore a weak nucleophile. Therefore, if we use NaI, a substitution reaction takes place ($S{N_2}$ ). In $S{N_2}$ mechanism, the incoming nucleophile (${I^ - }$ ) approaches in a direction opposite to the leaving group ( $B{r^ - }$ ) and a bond is made and broken simultaneously involving a five-membered transition state. This mechanism occurs in substitution reactions in primary alkyl halides. The bromide in bromopropane will be replaced by iodide ion. We get iodopropane as a product instead of an alkene. It does not favour elimination reactions. The reaction is as follows:

The right option is $(1)$ NaI.
Note :
The carbon atom to which the functional group is added, is called as $\alpha $ carbon and the immediate neighbouring carbon atom attached to $\alpha $ carbon is known as $\beta $ carbon. Hydrogen atoms attached to $\alpha $ carbon are called $\alpha $ hydrogen, and those attached to $\beta $ carbon are called $\beta $ hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




