
Which of the following products is formed when $1,2,4 - {\text{trimethylcyclopentene}}$ is hydrogenated?
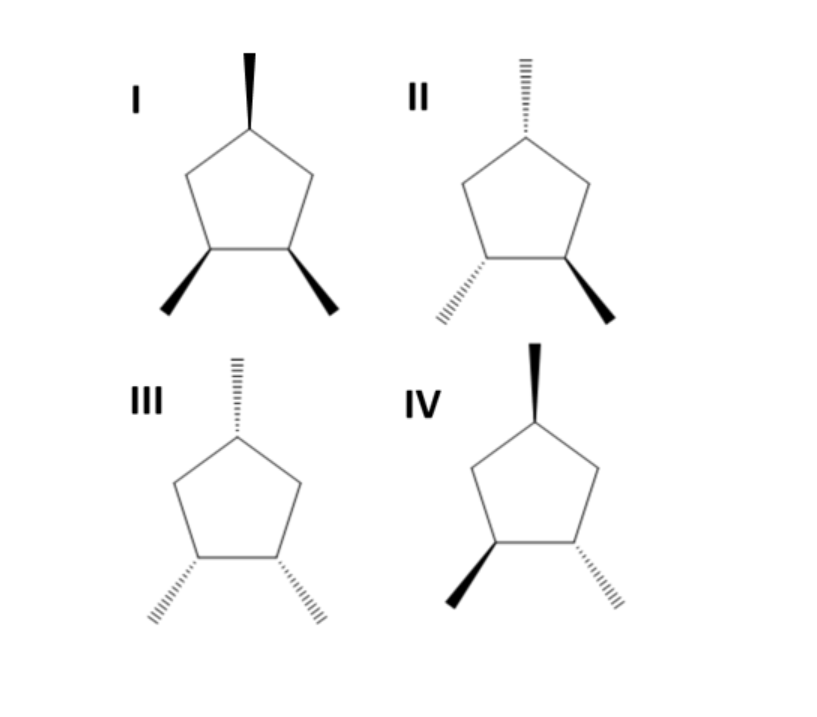
(A) I
(B) I and III
(C) II
(D) II and IV

Answer
521.7k+ views
Hint: Hydrogenation is a chemical reaction which occurs between hydrogen molecules and any other compound or element. Hydrogenation reaction takes place in the presence of catalysts such as palladium, nickel, platinum, etc. This reaction is an example of exothermic reaction.
Complete answer:
Hydrogenation is an additional reaction between ${H_2}$ and other compounds usually in the presence of a catalyst. The pair of hydrogen atoms in the hydrogen molecule carry out this process. Hydrogenation is used in the food industry too. It improves flavour stability. Trimethylcyclopentene consists of three methyl groups, a double bond and has a cyclic structure. The hydrogenation reaction of $1,2,4 - {\text{trimethylcyclopentene}}$is shown below:
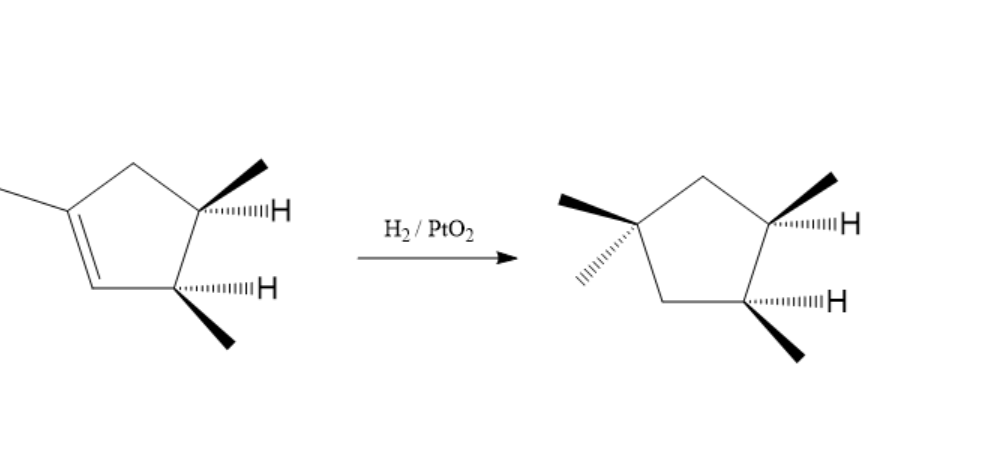
In the above reaction, $Pt{O_2}$ acts as a catalyst. In hydrogenation reaction, two atoms of hydrogen molecule are added to double bond present and as a result saturated alkane is formed. The product is more stable. So, the reaction is thermodynamically favourable. Here, hydrogen is added to the opposite side of the methyl group to avoid steric hindrance.
Therefore, option A is correct.
Note:
$Pt{O_2}$ is platinum oxide which is also called Adams catalyst. It is dark brown in color and appears in powdered form. It is not soluble in water, but it is soluble in acid, alcohol, etc. When an alkyne undergoes hydrogenation reaction, a cis-alkene is formed. It should be noted that reduction of alkenes can be performed with Adams catalyst in the presence of nitro groups without reducing those nitro groups.
Complete answer:
Hydrogenation is an additional reaction between ${H_2}$ and other compounds usually in the presence of a catalyst. The pair of hydrogen atoms in the hydrogen molecule carry out this process. Hydrogenation is used in the food industry too. It improves flavour stability. Trimethylcyclopentene consists of three methyl groups, a double bond and has a cyclic structure. The hydrogenation reaction of $1,2,4 - {\text{trimethylcyclopentene}}$is shown below:

In the above reaction, $Pt{O_2}$ acts as a catalyst. In hydrogenation reaction, two atoms of hydrogen molecule are added to double bond present and as a result saturated alkane is formed. The product is more stable. So, the reaction is thermodynamically favourable. Here, hydrogen is added to the opposite side of the methyl group to avoid steric hindrance.
Therefore, option A is correct.
Note:
$Pt{O_2}$ is platinum oxide which is also called Adams catalyst. It is dark brown in color and appears in powdered form. It is not soluble in water, but it is soluble in acid, alcohol, etc. When an alkyne undergoes hydrogenation reaction, a cis-alkene is formed. It should be noted that reduction of alkenes can be performed with Adams catalyst in the presence of nitro groups without reducing those nitro groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




