
Which of the following processes does not involve oxidation of iron?
(A)- Formation of $Fe{{(CO)}_{5}}$ from Fe
(B)- Liberation of ${{H}_{2}}$ from steam by iron at high temperature
(C)- Rusting of iron sheets
(D)- Decolourization of the blue $CuS{{O}_{4}}$ solution by iron
Answer
577.8k+ views
Hint: The loss of electrons during a chemical reaction by a molecule, atom or ion is known as Oxidation. When the oxidation state of a molecule, atom or ion is increased, it means that oxidation has occurred. The opposite to oxidation is the reduction in which a gain of electrons or the oxidation state of an atom, molecule or ion is decreased.
Complete answer:
-Let us understand the process of oxidation taking iron as an example.
-When an iron object is subjected to oxidation, it undergoes loss of electrons. Unoxidized ion is a strong and structurally sound metal, whereas oxidized iron is brittle and reddish powder.
-The diagram below illustrated the process of oxidation in an atom of iron-
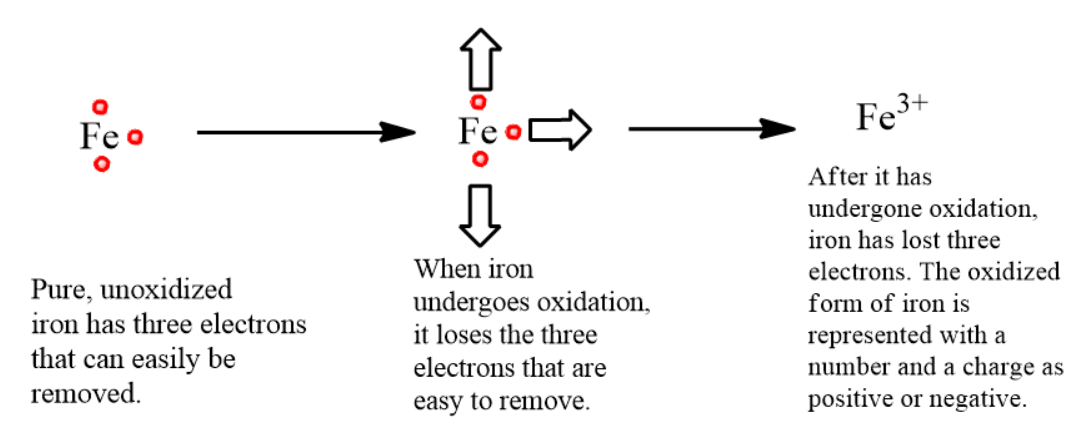
-One when the iron has undergone oxidation, it now carries a positive charge due to loss of three electrons which is represented as a superscript to the right of the iron symbol with number three and a positive sign.
-Iron is very easily oxidized, hence it is very important to minimize the exposure of iron to oxygen and moisture otherwise will continue losing electrons to oxygen as long as oxygen is present.
-Let us now consider each option one by one to examine whether the oxidation is occurring or not.
-In option A, the formation of $Fe{{(CO)}_{5}}$ Fe. The reaction for this is given as-
$Fe+5CO\to Fe{{(CO)}_{5}}$
The oxidation state of iron in the reactant is zero and in the product also zero. Since there is no increase in the oxidation state of iron, therefore it is not an oxidation process.
-In option B, the liberation of ${{H}_{2}}$ from steam by iron at high temperature is mentioned. As we know that liberation of oxygen is an oxidation process which adds oxygen molecules and removes hydrogen from the compound. Hence it is an example of oxidation.
-In option C, rusting of iron is mentioned. As mentioned above, iron in presence of oxygen liberates electron forming iron oxide whose reaction can be given as-
$4Fe+3{{O}_{3}}+6{{H}_{2}}O\to 4Fe{{(OH)}_{3}}$
The oxidation of iron is increased from 0 to +3 which implies it is oxidation.
-In option D, decolourization of the blue $CuS{{O}_{4}}$ solution by iron. When iron nails are immersed in copper sulphate solution, the iron element being stronger than copper displaces it and forms ferrous sulphate due to which decolourization of copper sulphate occurs. The reaction for it is given as –
$CuS{{O}_{4}}+Fe\to FeS{{O}_{4}}+Cu$
The oxidation state of iron in this displacement reaction is increased from zero to +2, indicating this and an oxidation reaction.
Therefore, the correct answer is option A.
Note:
The oxidation of iron forms iron oxides which are of great significance in the natural environment. Many hydroxides such as ferric hydroxide and oxyhydroxides are precursors of pure and complex oxides. Ferrites are essential ferromagnetic materials. Among all the ferrites, the iron oxides and ferrite have shown magnetic nanoparticles with appropriate surface chemistry for the preparation by wet chemical methods such as colloid chemical or sol-gel methods or by dry processes such as vapour deposition techniques.
Complete answer:
-Let us understand the process of oxidation taking iron as an example.
-When an iron object is subjected to oxidation, it undergoes loss of electrons. Unoxidized ion is a strong and structurally sound metal, whereas oxidized iron is brittle and reddish powder.
-The diagram below illustrated the process of oxidation in an atom of iron-

-One when the iron has undergone oxidation, it now carries a positive charge due to loss of three electrons which is represented as a superscript to the right of the iron symbol with number three and a positive sign.
-Iron is very easily oxidized, hence it is very important to minimize the exposure of iron to oxygen and moisture otherwise will continue losing electrons to oxygen as long as oxygen is present.
-Let us now consider each option one by one to examine whether the oxidation is occurring or not.
-In option A, the formation of $Fe{{(CO)}_{5}}$ Fe. The reaction for this is given as-
$Fe+5CO\to Fe{{(CO)}_{5}}$
The oxidation state of iron in the reactant is zero and in the product also zero. Since there is no increase in the oxidation state of iron, therefore it is not an oxidation process.
-In option B, the liberation of ${{H}_{2}}$ from steam by iron at high temperature is mentioned. As we know that liberation of oxygen is an oxidation process which adds oxygen molecules and removes hydrogen from the compound. Hence it is an example of oxidation.
-In option C, rusting of iron is mentioned. As mentioned above, iron in presence of oxygen liberates electron forming iron oxide whose reaction can be given as-
$4Fe+3{{O}_{3}}+6{{H}_{2}}O\to 4Fe{{(OH)}_{3}}$
The oxidation of iron is increased from 0 to +3 which implies it is oxidation.
-In option D, decolourization of the blue $CuS{{O}_{4}}$ solution by iron. When iron nails are immersed in copper sulphate solution, the iron element being stronger than copper displaces it and forms ferrous sulphate due to which decolourization of copper sulphate occurs. The reaction for it is given as –
$CuS{{O}_{4}}+Fe\to FeS{{O}_{4}}+Cu$
The oxidation state of iron in this displacement reaction is increased from zero to +2, indicating this and an oxidation reaction.
Therefore, the correct answer is option A.
Note:
The oxidation of iron forms iron oxides which are of great significance in the natural environment. Many hydroxides such as ferric hydroxide and oxyhydroxides are precursors of pure and complex oxides. Ferrites are essential ferromagnetic materials. Among all the ferrites, the iron oxides and ferrite have shown magnetic nanoparticles with appropriate surface chemistry for the preparation by wet chemical methods such as colloid chemical or sol-gel methods or by dry processes such as vapour deposition techniques.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




