
Which of the following primary amine is not prepared by Gabriel phthalimide synthesis:
A.\[C{H_3} - C{H_2} - N{H_2}\]
B.\[C{H_3} - N{H_2}\]
C.\[Ph - N{H_2}\]
D.\[Ph - C{H_2} - N{H_2}\]
Answer
510.3k+ views
Hint: The Gabriel synthesis is a chemical reaction that transforms primary alkyl halides into primary amines. Traditionally, the reaction uses potassium phthalimide and, therefore, is also called Gabriel phthalimide synthesis. It uses a nucleophilic substitution reaction to form primary amines from alkyl halide.
Complete answer:
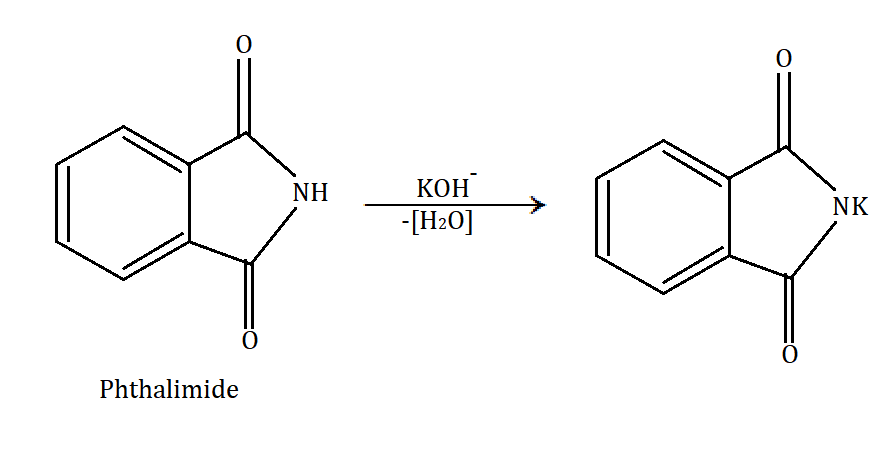
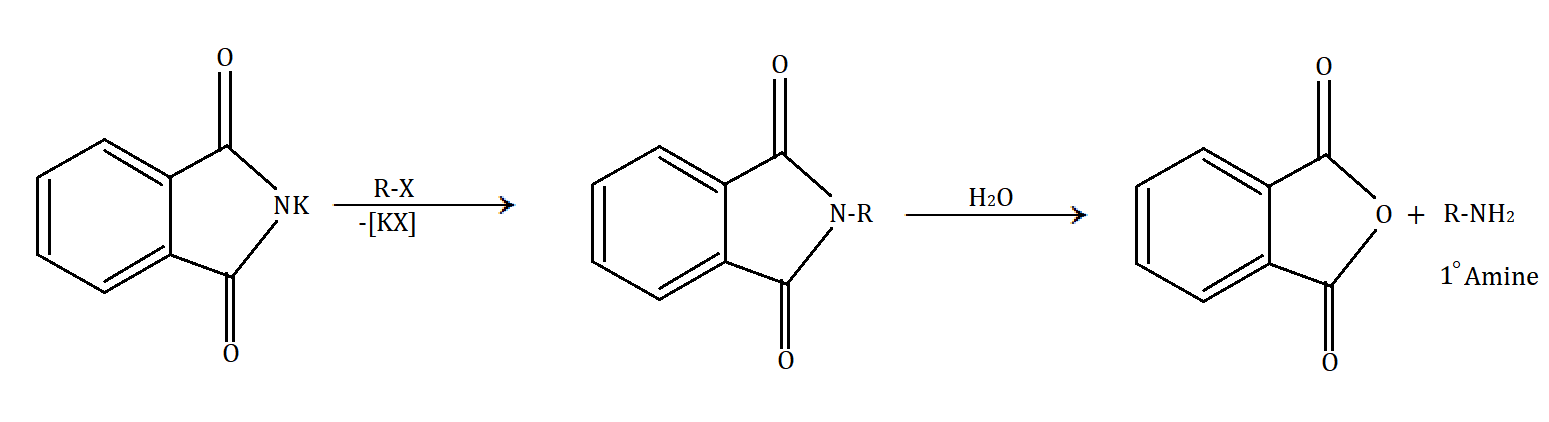
In Gabriel phthalimide synthesis, a base abstract proton from phthalimide gives a nucleophile phthalimide ion which attacks on the unhindered primary alkyl halide. The base hydrolysis of alkylated phthalimide gives the primary unhindered amine and phthalimide ion. Phthalimide ion itself is a bulky ion, so it prefers attack on hindered alkyl halide.
Gabriel phthalimide synthesis can be understood through the following mechanism:
In the given options, \[C{H_3} - C{H_2} - N{H_2}\] (ethyl amine), \[C{H_3} - N{H_2}\] (methyl amine) and \[Ph - C{H_2} - N{H_2}\] (\[1 - \]phenyl methanamine) are the compounds which can be prepared by Gabriel phthalimide synthesis. Ethylamine and methylamine are simple aliphatic compounds. While in \[1 - \]phenyl methanamine, the amine part is at the methyl part and phenyl is acting as a functional group and hence should be considered an aliphatic amine.
Hence, \[Ph - N{H_2}\] (aniline) is the only compound that cannot be prepared by Gabriel phthalimide synthesis.
Therefore, the correct option is C.
Note:
Gabriel phthalimide synthesis is only used for primary amines as steric hindrances do not allow the reaction for secondary and tertiary aliphatic amines. It is also not applicable for \[1^\circ \] aromatic compounds, aryl halide does not undergo nucleophilic substitution reaction. Aromatic compounds usually show electrophilic substitution reactions. Nucleophilic substitution destabilizes the resonance of aromatic compounds.
Complete answer:
In Gabriel phthalimide synthesis, a base abstract proton from phthalimide gives a nucleophile phthalimide ion which attacks on the unhindered primary alkyl halide. The base hydrolysis of alkylated phthalimide gives the primary unhindered amine and phthalimide ion. Phthalimide ion itself is a bulky ion, so it prefers attack on hindered alkyl halide.
Gabriel phthalimide synthesis can be understood through the following mechanism:


In the given options, \[C{H_3} - C{H_2} - N{H_2}\] (ethyl amine), \[C{H_3} - N{H_2}\] (methyl amine) and \[Ph - C{H_2} - N{H_2}\] (\[1 - \]phenyl methanamine) are the compounds which can be prepared by Gabriel phthalimide synthesis. Ethylamine and methylamine are simple aliphatic compounds. While in \[1 - \]phenyl methanamine, the amine part is at the methyl part and phenyl is acting as a functional group and hence should be considered an aliphatic amine.
Hence, \[Ph - N{H_2}\] (aniline) is the only compound that cannot be prepared by Gabriel phthalimide synthesis.
Therefore, the correct option is C.
Note:
Gabriel phthalimide synthesis is only used for primary amines as steric hindrances do not allow the reaction for secondary and tertiary aliphatic amines. It is also not applicable for \[1^\circ \] aromatic compounds, aryl halide does not undergo nucleophilic substitution reaction. Aromatic compounds usually show electrophilic substitution reactions. Nucleophilic substitution destabilizes the resonance of aromatic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




