
Which of the following on hydrolysis with dilute alkali followed by an acidification gives benzaldehyde.
A.Benzotrichloride
B.Benzal chloride
C.Benzyl chloride
D.P-chlorotoluene
Answer
518.1k+ views
Hint: We have to know that, a substance response in which water is utilized to separate a compound; this is accomplished by breaking a covalent security in the compound by embedding a water particle across the security. Something contrary to this is a drying out buildup response.
Complete answer:
We have to know that benzotrichloride, otherwise called trichlorotoluene, is a natural compound with the recipe ${C_6}{H_5}CC{l_3}$ . It is mainly utilized as a middle of the road in the arrangement of other compound items like colors.
The immediate hydrolysis of benzotrichloride with water alone, to create benzoic corrosive and hydrochloric corrosive, can be affected at high temperatures.
Therefore, option (A) is incorrect.
We have to know that benzal chloride is a natural compound with the recipe ${C_6}{H_5}CHC{l_2}$ . This dreary fluid is a lachrymator and is utilized as a structure block in natural blend. Benzal chloride is hydrolyzed to give benzaldehyde.
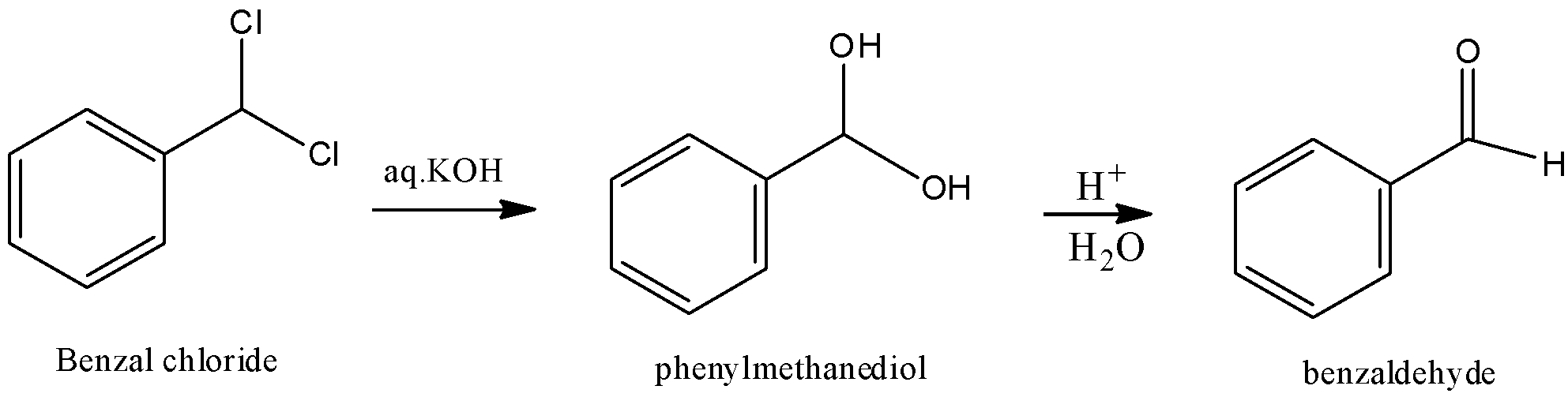
Therefore, option (B) is correct.
We have to know that benzyl chloride, or alpha-chlorotoluene, is a natural compound with the equation ${C_6}{H_5}C{H_2}Cl$ . This dry fluid is a responsive organochlorine compound that is a generally utilized substance building block. Benzyl chloride responds with water in a hydrolysis response to frame benzyl liquor and hydrochloric corrosive.
Therefore, option (C) is incorrect.
The chemical formula of p-chlorotoluene is ${C_7}{H_7}Cl$ . Therefore, option (D) is incorrect.
Therefore, option (B) is correct.
Note:
We have to know that acidification is a characteristic interaction by which the substance of the dirt turns out to be more acidic. This occurs through the deficiency of fundamental or alkalic components like calcium, magnesium, and potassium and additionally the presentation of acidic components like hydrogen and aluminum.
Complete answer:
We have to know that benzotrichloride, otherwise called trichlorotoluene, is a natural compound with the recipe ${C_6}{H_5}CC{l_3}$ . It is mainly utilized as a middle of the road in the arrangement of other compound items like colors.
The immediate hydrolysis of benzotrichloride with water alone, to create benzoic corrosive and hydrochloric corrosive, can be affected at high temperatures.
Therefore, option (A) is incorrect.
We have to know that benzal chloride is a natural compound with the recipe ${C_6}{H_5}CHC{l_2}$ . This dreary fluid is a lachrymator and is utilized as a structure block in natural blend. Benzal chloride is hydrolyzed to give benzaldehyde.

Therefore, option (B) is correct.
We have to know that benzyl chloride, or alpha-chlorotoluene, is a natural compound with the equation ${C_6}{H_5}C{H_2}Cl$ . This dry fluid is a responsive organochlorine compound that is a generally utilized substance building block. Benzyl chloride responds with water in a hydrolysis response to frame benzyl liquor and hydrochloric corrosive.
Therefore, option (C) is incorrect.
The chemical formula of p-chlorotoluene is ${C_7}{H_7}Cl$ . Therefore, option (D) is incorrect.
Therefore, option (B) is correct.
Note:
We have to know that acidification is a characteristic interaction by which the substance of the dirt turns out to be more acidic. This occurs through the deficiency of fundamental or alkalic components like calcium, magnesium, and potassium and additionally the presentation of acidic components like hydrogen and aluminum.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




