
Which of the following is the incorrect IUPAC name of a compound?
A. Pent-3-ene
B. Prop-1-en-2-yne
C. 1-methylpropane
D. All are incorrect.
Answer
573.9k+ views
Hint: The IUPAC system is the most widely used system of naming in organic chemistry and the most important feature of this system is that for any given molecular structure there exists one IUPAC name.
Complete step by step answer:
To find the incorrect IUPAC name of a compound, we have to draw the structure of all the given compounds. If we talk about option A i.e. pent -3-ene, as per the structure drawn below, there are five carbon chain and the position of the double bond is located on the third carbon:
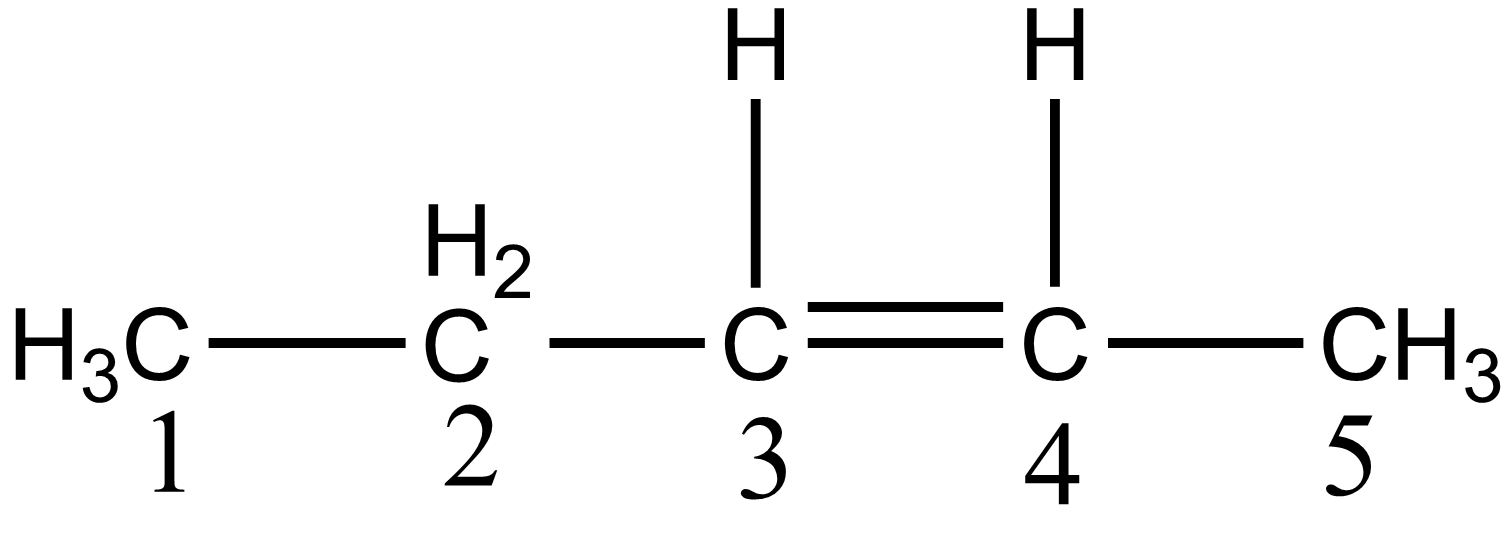
But according to the IUPAC rule, the numbering of parent chains is done from that side in which a double bond gets the lowest position. According to the IUPAC rule, its naming should be pent-2-ene which is given as:
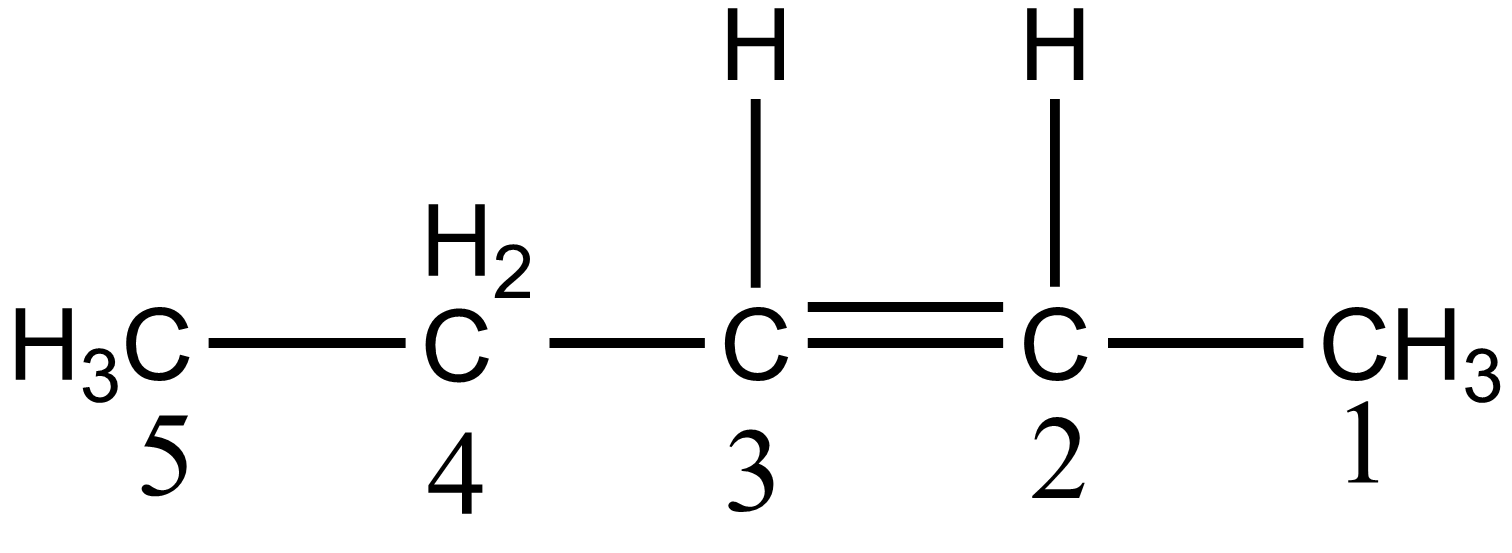
Hence option A is wrong.
In the case of option B, the structure of Prop-1-en-2-yne drawn below is not possible because the valency of carbon is four i.e. carbon makes maximum foy=ur bond but here five bonds are formed by carbon. Hence it is also wrong.:
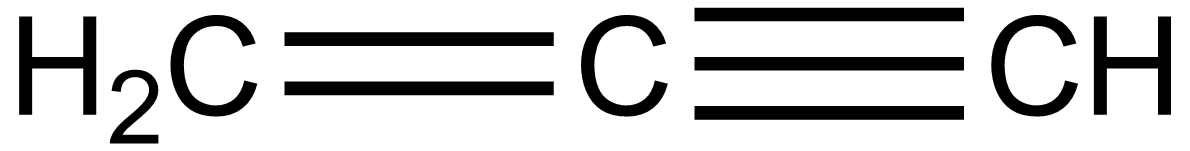
In the case of option C, 1-methylpropane, the structure is given below:
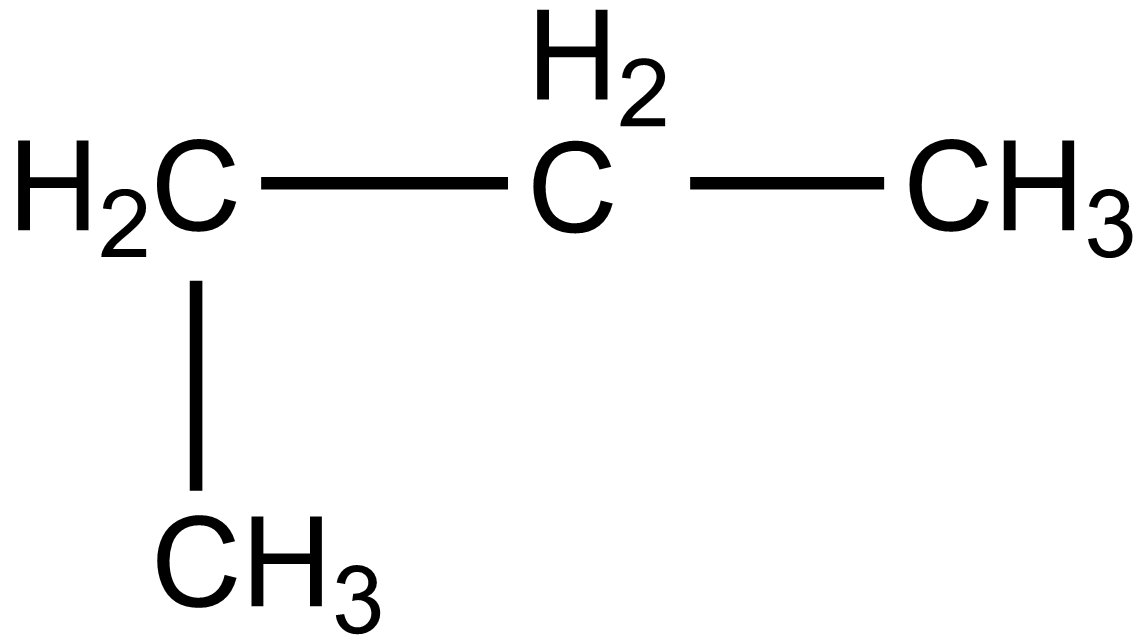
According to IUPAC the name of the above compound should be butane which is given as:
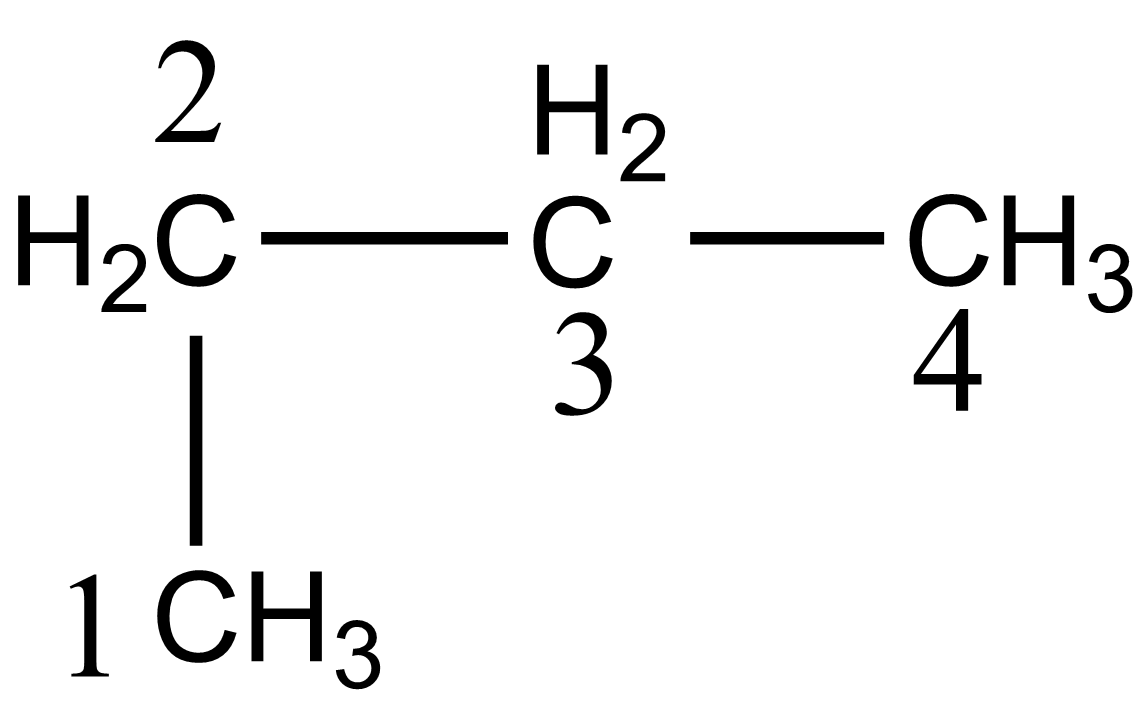
Hence option C is also incorrect.
So, the correct answer is Option D.
Note: The IUPAC name of any organic compound contains three parts:
1. Word root which indicates the number of carbon atoms in the parent chain. Thus it is the basic unit of the name.
2. Suffix: There are two types of a suffix such as primary and secondary suffix. Primary suffix indicates carbon is saturated or unsaturated whereas the secondary suffix indicates the nature of the functional group.
3. The prefix is also two types such as primary as well as a secondary prefix. Primary prefix distinguishes cyclic or acyclic compounds whereas secondary prefix indicates a functional group which is treated as a substitute.
Complete step by step answer:
To find the incorrect IUPAC name of a compound, we have to draw the structure of all the given compounds. If we talk about option A i.e. pent -3-ene, as per the structure drawn below, there are five carbon chain and the position of the double bond is located on the third carbon:

But according to the IUPAC rule, the numbering of parent chains is done from that side in which a double bond gets the lowest position. According to the IUPAC rule, its naming should be pent-2-ene which is given as:

Hence option A is wrong.
In the case of option B, the structure of Prop-1-en-2-yne drawn below is not possible because the valency of carbon is four i.e. carbon makes maximum foy=ur bond but here five bonds are formed by carbon. Hence it is also wrong.:
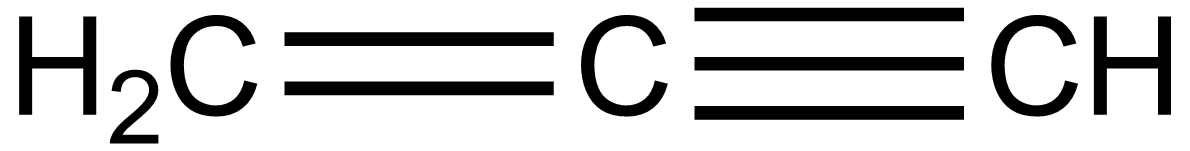
In the case of option C, 1-methylpropane, the structure is given below:

According to IUPAC the name of the above compound should be butane which is given as:

Hence option C is also incorrect.
So, the correct answer is Option D.
Note: The IUPAC name of any organic compound contains three parts:
1. Word root which indicates the number of carbon atoms in the parent chain. Thus it is the basic unit of the name.
2. Suffix: There are two types of a suffix such as primary and secondary suffix. Primary suffix indicates carbon is saturated or unsaturated whereas the secondary suffix indicates the nature of the functional group.
3. The prefix is also two types such as primary as well as a secondary prefix. Primary prefix distinguishes cyclic or acyclic compounds whereas secondary prefix indicates a functional group which is treated as a substitute.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




