
Which of the following is not possible?
A. $ S{F_4} $
B. $ O{F_2} $
C. $ O{F_4} $
D. $ {O_2}{F_2} $
Answer
559.5k+ views
Hint: The possibility of a particular molecule is defined according to the stability of the particular molecule with respect to the structure attaining the stable octet configuration. In the given conditions, there can be different geometry of the elements but the stability depends on the bond formation and the electrons involved in bond formation to reach the stable state.
Complete answer
Here all the given compounds that are listed are formed with two-non-metals. The bond formation between the non-metals have a specific character of forming covalent structures because the valence shell has few electrons less than the octet configuration and that is why the sharing of electrons is the process on which bond formation occurs.
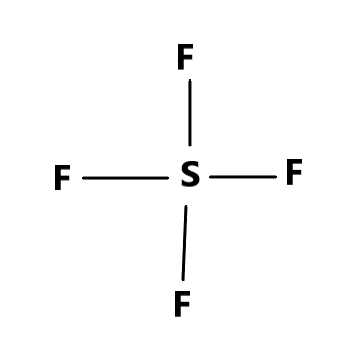
The first compound is $ S{F_4} $ which is theoretically possible because Sulphur has $ 4 $ valence electrons, while the Fluorine has $ 7 $ valence electrons. Therefore, electron sharing takes place between these two elements.
The second compound is $ O{F_2} $ which is also theoretically possible as there are $ 6 $ valence electrons in the atom of $ O $ and each of the atom Fluorine has $ 7 $ valence electrons. Therefore, the electron sharing forming two covalent bonds between oxygen and the two fluorine atoms can form the given molecule.
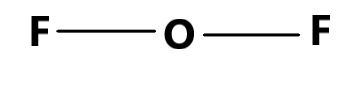
The fourth compound is $ {O_2}{F_2} $ and that can be formed with the specific atoms because they can together form the covalently bonded structure. This offers a stable compound structure for the given molecule and the stable octet form of all the atoms is reached.
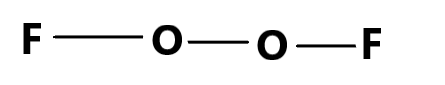
But the compound $ O{F_4} $ is not possible to be formed theoretically as the valence electrons in the Oxygen atom is $ 6 $ and that is why if four fluorine atoms are associated, the octet configuration will be lost and that is why a stable compound will not be produced. Therefore, the given compound that is theoretically not possible is C. $ O{F_4} $ .
Note
The compounds require stability to exist in nature and that can only be attained if the configuration of electrons involved in the compound formation offers that stability. The elements that are involved in the formation of a compound plays a major role in this process because the stability of each atom is important after formation of bonds.
Complete answer
Here all the given compounds that are listed are formed with two-non-metals. The bond formation between the non-metals have a specific character of forming covalent structures because the valence shell has few electrons less than the octet configuration and that is why the sharing of electrons is the process on which bond formation occurs.
The first compound is $ S{F_4} $ which is theoretically possible because Sulphur has $ 4 $ valence electrons, while the Fluorine has $ 7 $ valence electrons. Therefore, electron sharing takes place between these two elements.

The second compound is $ O{F_2} $ which is also theoretically possible as there are $ 6 $ valence electrons in the atom of $ O $ and each of the atom Fluorine has $ 7 $ valence electrons. Therefore, the electron sharing forming two covalent bonds between oxygen and the two fluorine atoms can form the given molecule.
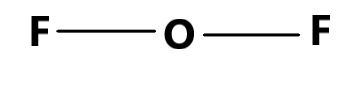
The fourth compound is $ {O_2}{F_2} $ and that can be formed with the specific atoms because they can together form the covalently bonded structure. This offers a stable compound structure for the given molecule and the stable octet form of all the atoms is reached.
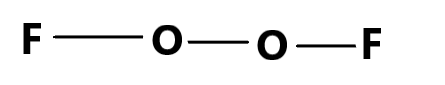
But the compound $ O{F_4} $ is not possible to be formed theoretically as the valence electrons in the Oxygen atom is $ 6 $ and that is why if four fluorine atoms are associated, the octet configuration will be lost and that is why a stable compound will not be produced. Therefore, the given compound that is theoretically not possible is C. $ O{F_4} $ .
Note
The compounds require stability to exist in nature and that can only be attained if the configuration of electrons involved in the compound formation offers that stability. The elements that are involved in the formation of a compound plays a major role in this process because the stability of each atom is important after formation of bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




