
Which of the following is not aromatic?
(A) Benzene
(B) Naphthalene
(C) Pyridine
(D) $ 1,3,5 - $ heptatriene
Answer
492.9k+ views
Hint: Aromaticity of any compound is determined by applying Huckle’s rule which states that if any compound possesses $ \left( {4n + 2} \right)\pi $ electrons which have capability to delocalize are known as aromatic compounds.
Complete Step By Step Answer:
In order to calculate the aromaticity of a compound we begin with determining the number of electrons present in it. After this, put the value of $ \left( \pi \right) $ electrons in Huckle’s formula. If the final answer is a whole number then the compound must be aromatic.
Structure of benzene is:
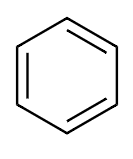
From the above structure we find that there are a total $ 6\pi $ electrons present in benzene. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 6 $
$ 4n = 6 - 2 $
After solving this we get
$ n = \dfrac{4}{4} $
$ n = 1 $
Since, the value of $ \left( n \right) $ is a whole number, benzene is an aromatic compound.
Structure of Naphthalene is:
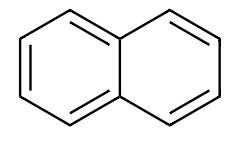
From the above structure we find that there are a total $ 10\pi $ electrons present in naphthalene. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 10 $
$ 4n = 10 - 2 $
After solving this we get
$ n = \dfrac{8}{4} $
$ n = 2 $
Since, the value of $ \left( n \right) $ is a whole number, naphthalene is an aromatic compound.
Structure of pyridine is:
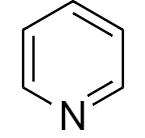
From the above structure we find that there are a total $ 6\pi $ electrons present in pyridine. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 6 $
$ 4n = 6 - 2 $
After solving this we get
$ n = \dfrac{4}{4} $
$ n = 1 $
Since, the value of $ \left( n \right) $ is a whole number, pyridine is an aromatic compound.
Structure of $ 1,3,5 - $ heptatriene is:
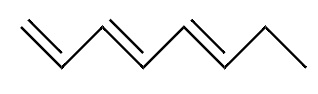
From the above structure we see that it is not a cyclic structure. therefore $ 1,3,5 - $ heptatriene fails to fulfill the basic requirement of aromaticity. Hence, $ 1,3,5 - $ heptatriene is not aromatic.
$ \Rightarrow $ $ 1,3,5 - $ heptatriene is not an aromatic compound. Therefore option $ \left( {iv} \right) $ is the correct option.
Note:
Due to the presence of aroma or pleasant smell from the compound they are known as aromatic. According to Huckle’s rule a compound is said to be aromatic if it satisfies the basic requirement like compound must be planar, cyclic, and have $ \left( {4n + 2} \right)\pi $ delocalized electrons.
Complete Step By Step Answer:
In order to calculate the aromaticity of a compound we begin with determining the number of electrons present in it. After this, put the value of $ \left( \pi \right) $ electrons in Huckle’s formula. If the final answer is a whole number then the compound must be aromatic.
Structure of benzene is:

From the above structure we find that there are a total $ 6\pi $ electrons present in benzene. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 6 $
$ 4n = 6 - 2 $
After solving this we get
$ n = \dfrac{4}{4} $
$ n = 1 $
Since, the value of $ \left( n \right) $ is a whole number, benzene is an aromatic compound.
Structure of Naphthalene is:

From the above structure we find that there are a total $ 10\pi $ electrons present in naphthalene. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 10 $
$ 4n = 10 - 2 $
After solving this we get
$ n = \dfrac{8}{4} $
$ n = 2 $
Since, the value of $ \left( n \right) $ is a whole number, naphthalene is an aromatic compound.
Structure of pyridine is:

From the above structure we find that there are a total $ 6\pi $ electrons present in pyridine. On applying Huckle’s rule we get,
$ \left( {4n + 2} \right) = 6 $
$ 4n = 6 - 2 $
After solving this we get
$ n = \dfrac{4}{4} $
$ n = 1 $
Since, the value of $ \left( n \right) $ is a whole number, pyridine is an aromatic compound.
Structure of $ 1,3,5 - $ heptatriene is:

From the above structure we see that it is not a cyclic structure. therefore $ 1,3,5 - $ heptatriene fails to fulfill the basic requirement of aromaticity. Hence, $ 1,3,5 - $ heptatriene is not aromatic.
$ \Rightarrow $ $ 1,3,5 - $ heptatriene is not an aromatic compound. Therefore option $ \left( {iv} \right) $ is the correct option.
Note:
Due to the presence of aroma or pleasant smell from the compound they are known as aromatic. According to Huckle’s rule a compound is said to be aromatic if it satisfies the basic requirement like compound must be planar, cyclic, and have $ \left( {4n + 2} \right)\pi $ delocalized electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




