
Which of the following is not an acidic salt?
A.${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$
B.${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$
C.${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$
D.All of these
Answer
589.2k+ views
Hint: Any salt that has replaceable hydrogen atoms is known as an acidic salt. The aqueous solutions of acidic salts turn blue litmus red. Thus, the aqueous solutions of acidic salts are acidic in nature.
Step by step answer:
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is as follows:
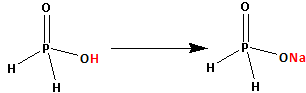
Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ has only one replaceable hydrogen atom. This one hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is formed.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is not an acidic salt.
Thus, option (A) is correct.
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ is as follows:
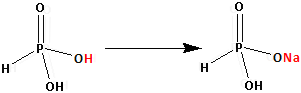
Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ has two replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is formed. One replaceable hydrogen atom is still remaining.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is an acidic salt.
Thus, option (B) is not correct.
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ is as follows:
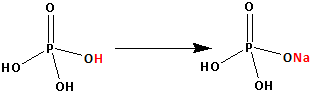
Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ has three replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is formed. Two replaceable hydrogen atoms are still remaining.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is an acidic salt.
Thus, option (C) is not correct.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is not an acidic salt.
Thus, the correct option is (A). ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$.
Note: In the acids, the hydrogen atoms that are attached to the oxygen atom are known as replaceable hydrogen atoms. Hydrogen atoms attached to phosphorus directly are not replaceable.
Step by step answer:
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is as follows:

Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_{\text{2}}}$ has only one replaceable hydrogen atom. This one hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is formed.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is not an acidic salt.
Thus, option (A) is correct.
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ is as follows:

Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_3}$ has two replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is formed. One replaceable hydrogen atom is still remaining.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_3}$ is an acidic salt.
Thus, option (B) is not correct.
The salt ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is formed by the neutralization of weak acid ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ and strong base ${\text{NaOH}}$.
The structure of ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ is as follows:

Thus, ${{\text{H}}_{\text{3}}}{\text{P}}{{\text{O}}_4}$ has three replaceable hydrogen atom. One hydrogen atom is replaced by one sodium atom and ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is formed. Two replaceable hydrogen atoms are still remaining.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_4}$ is an acidic salt.
Thus, option (C) is not correct.
Thus, ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$ is not an acidic salt.
Thus, the correct option is (A). ${\text{Na}}{{\text{H}}_{\text{2}}}{\text{P}}{{\text{O}}_{\text{2}}}$.
Note: In the acids, the hydrogen atoms that are attached to the oxygen atom are known as replaceable hydrogen atoms. Hydrogen atoms attached to phosphorus directly are not replaceable.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




