
Which of the following is not a unit of pressure?
A. Pascal
B. Torr
C. Dynes
D. Atm
Answer
582.6k+ views
Hint: Pressure is defined as the force acting perpendicular to the plane of a surface, on a unit area of the surface. Find out the SI unit of pressure. Also find the other units of pressure and figure out which one of the options is not a unit of pressure.
Formula used:
$\text{Pressure = }\dfrac{\text{perpendicular force}}{\text{area}}$
Complete answer:
Pressure is defined as the force acting perpendicular to the plane of a surface, on a unit area of the surface. If the normal force acting on the surface is uniform then the pressure on the surface is simply force upon the area of the surface perpendicular to the force.
i.e. $\text{Pressure = }\dfrac{\text{perpendicular force}}{\text{area}}$ .
For example, if a perpendicular force of 10N is acting on one side of cube with surface area of each side equal to $5{{m}^{2}}$ then the pressure (P) exerted on it is $P=\dfrac{\text{perpendicular force}}{\text{area}}=\dfrac{10}{5}=2N{{m}^{-2}}$.
We can also depict this by a diagram as shown.
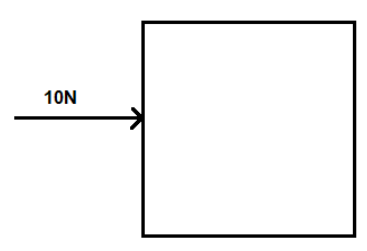
If the force is not normal to the plane of the surface then we take the component normal to the plane into consideration.
To understand this, consider the same example given above except this time the force not perpendicular to the surface of the side. The force is acting at an angle $\theta $ (as shown below).
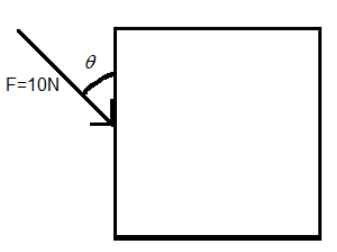
In this case, we have to consider that component of the force, which is perpendicular to the surface. For this we have to resolve the force into its components (as shown). By geometry the components of the force F will be $F\cos \theta $ and $F\sin \theta $.
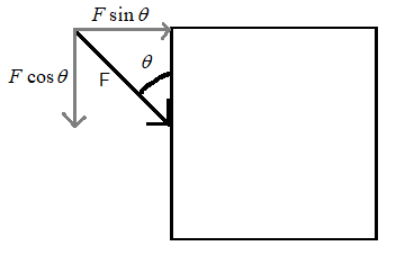
Hence, the perpendicular force to the surface is $F\sin \theta $. Therefore, in this case the pressure on the side of the cube is $\dfrac{F\cos \theta }{A}=\dfrac{10\cos \theta }{5}=2\cos \theta N{{m}^{-2}}$.
The SI unit of pressure is called pascal. That is 1$N{{m}^{-2}}$ is equal to 1 pascal.
The other units of pressure are torr, mmHg atm. These units of pressure are used for the pressure of gases.
Out of the four options, we know that pascal, torr and atm are units of pressure. Therefore, dynes is not a unit of pressure.
Dyne is a unit of force.
Hence, the correct option is C.
Note:
While calculating pressure only the component of the force that is normal to the surface, on which it is acting, is taken. Therefore, the direction of the original force does not matter. We need to know only the magnitude of the component of the force normal to the surface. Therefore, pressure does not have any specific direction. Hence, it is a scalar quantity.
Formula used:
$\text{Pressure = }\dfrac{\text{perpendicular force}}{\text{area}}$
Complete answer:
Pressure is defined as the force acting perpendicular to the plane of a surface, on a unit area of the surface. If the normal force acting on the surface is uniform then the pressure on the surface is simply force upon the area of the surface perpendicular to the force.
i.e. $\text{Pressure = }\dfrac{\text{perpendicular force}}{\text{area}}$ .
For example, if a perpendicular force of 10N is acting on one side of cube with surface area of each side equal to $5{{m}^{2}}$ then the pressure (P) exerted on it is $P=\dfrac{\text{perpendicular force}}{\text{area}}=\dfrac{10}{5}=2N{{m}^{-2}}$.
We can also depict this by a diagram as shown.

If the force is not normal to the plane of the surface then we take the component normal to the plane into consideration.
To understand this, consider the same example given above except this time the force not perpendicular to the surface of the side. The force is acting at an angle $\theta $ (as shown below).

In this case, we have to consider that component of the force, which is perpendicular to the surface. For this we have to resolve the force into its components (as shown). By geometry the components of the force F will be $F\cos \theta $ and $F\sin \theta $.

Hence, the perpendicular force to the surface is $F\sin \theta $. Therefore, in this case the pressure on the side of the cube is $\dfrac{F\cos \theta }{A}=\dfrac{10\cos \theta }{5}=2\cos \theta N{{m}^{-2}}$.
The SI unit of pressure is called pascal. That is 1$N{{m}^{-2}}$ is equal to 1 pascal.
The other units of pressure are torr, mmHg atm. These units of pressure are used for the pressure of gases.
Out of the four options, we know that pascal, torr and atm are units of pressure. Therefore, dynes is not a unit of pressure.
Dyne is a unit of force.
Hence, the correct option is C.
Note:
While calculating pressure only the component of the force that is normal to the surface, on which it is acting, is taken. Therefore, the direction of the original force does not matter. We need to know only the magnitude of the component of the force normal to the surface. Therefore, pressure does not have any specific direction. Hence, it is a scalar quantity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




