
Which of the following is not a form of carbon?
(A) Graphite
(B) Fullerene
(C) Microtubules
(D) Diamond
Answer
522.5k+ views
Hint: Carbon is found in many forms in nature. These forms are known as allotropes of carbon. These forms differ from each other in many physical properties.
Complete step by step solution:
First we will define Allotropy.
> Allotropy is defined as the property of an element to exist in different physical forms in the same state. This arises due to the difference in structure of the various forms of the element. Carbon exists in three different allotropic forms. Each form is known as an allotrope of the element.
> The three different allotropes of carbon are:
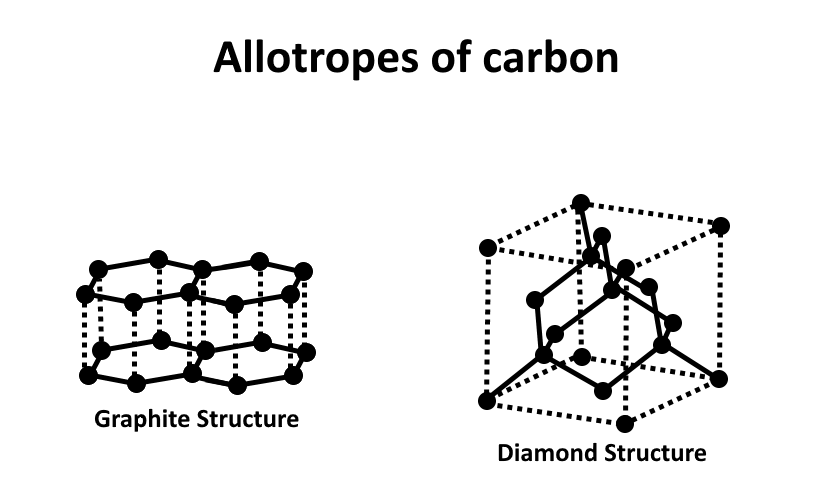
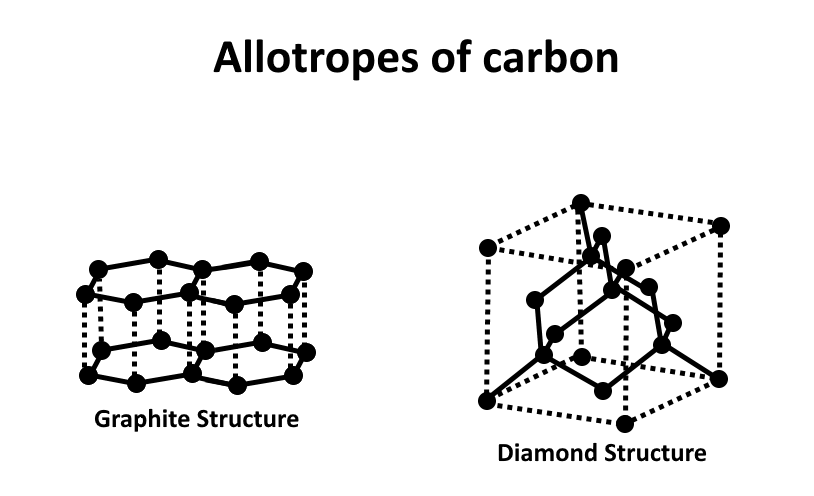
1) Diamond: In this form carbon is tetrahedral bonded to other carbon atoms. It is a precious element. Is formed in extreme temperature and pressure conditions.
2) Graphite: In this form carbon is present in hexagonal layers held together by weak Vander waals forces. It is greyish in colour and conducts electricity.
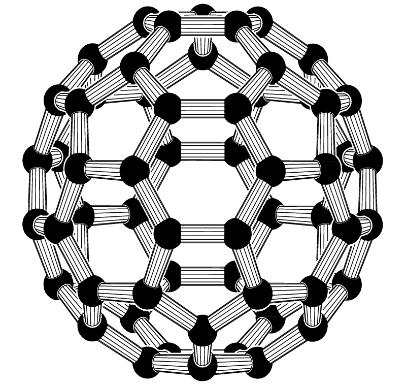
3) Fullerene: This form of carbon exists in large spherical molecules containing 60 carbon atoms. These are also known as buckminsterfullerene or buckyballs. They have a football like arrangement of carbon atoms.
Microtubule is a polymer. It forms the tubulin part of the cytoskeleton. It is not an allotrope of carbon.
So, we have seen that microtubule is the only option which is not an allotrope of carbon.
Hence, the answer to the given question is option (C).
Note: Carbon shows the maximum number of allotropes. This is because of its tetravalency and catenation power. Catenation means property to form long chains of compounds and tetravalency means sharing of 4 electrons to form bonds.
Complete step by step solution:
First we will define Allotropy.
> Allotropy is defined as the property of an element to exist in different physical forms in the same state. This arises due to the difference in structure of the various forms of the element. Carbon exists in three different allotropic forms. Each form is known as an allotrope of the element.
> The three different allotropes of carbon are:
1) Diamond: In this form carbon is tetrahedral bonded to other carbon atoms. It is a precious element. Is formed in extreme temperature and pressure conditions.
2) Graphite: In this form carbon is present in hexagonal layers held together by weak Vander waals forces. It is greyish in colour and conducts electricity.
3) Fullerene: This form of carbon exists in large spherical molecules containing 60 carbon atoms. These are also known as buckminsterfullerene or buckyballs. They have a football like arrangement of carbon atoms.
Microtubule is a polymer. It forms the tubulin part of the cytoskeleton. It is not an allotrope of carbon.
So, we have seen that microtubule is the only option which is not an allotrope of carbon.
Hence, the answer to the given question is option (C).
Note: Carbon shows the maximum number of allotropes. This is because of its tetravalency and catenation power. Catenation means property to form long chains of compounds and tetravalency means sharing of 4 electrons to form bonds.



Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




