
Which of the following is gem-dihalide:
(A)
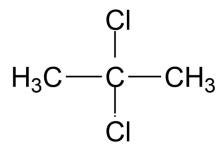
(B)
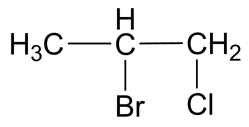
(C)
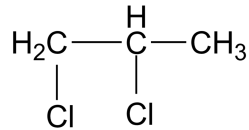
(D)
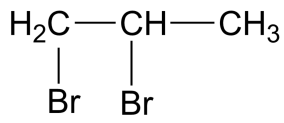




Answer
591k+ views
Hint: The organic compounds in which two halogens are attached to the same carbon atom are known as Geminal dihalides. (Gem means attached to the same atom and halide are the general term for halogens.)
Complete step by step solution:
-The word gem dihalide is an abbreviation for geminal dihalide.
-Geminal word in chemistry is derived from a Latin word ‘Gemini’ which means ‘twins’ which refers to the relationship between two atoms or functional groups which are attached to the same atoms.
-The common name for geminal dihalide is alkylidene halides.
-The IUPAC name for the compound in the first option is 2,2 dichloro propane. This compound was shown to produce acute toxicity in rats when subjected to inhalation and have affected sense organs.
-The IUPAC name for the compound in the second option is 1-chloro 2-bromo propane. This compound is sometimes used to determine the enantiomeric resolution of simple chromatographic methods.
-The IUPAC name for the compound in the third option is 1,2-dichloro propane. This compound is also known as propylene dichloride. This is an intermediate formed in the production of perchloroethylene and other chlorinated chemicals. Propylene dichloride was once used as a soil fumigant, chemical intermediate, and as an industrial solvent, but some of these uses have been discontinued. This compound was found to show carcinogenicity. In Japan, there were cases registered in March 2013 for cancer caused by the use of cleaning agents containing 1.2 dichloropropane.
-The IUPAC name for compound in the fourth option is 1,2-dibromo propane which is also known as propylene dibromide which is the simplest carbon hydrocarbon containing two bromine atoms.
So, the correct answer is option (A).
Note: You may get confused between geminal dihalide and vicinal dihalides. The geminal dihalides are organic compounds containing two halide groups attached to the same carbon whereas vicinal dihalides are organic compounds having two halide groups attached to two adjacent carbon atoms of the same chemical compound. The terms ‘geminal’ and ‘vicinal’ are used for chemical compounds having substituents. These terms distinguish chemical compounds based on the location of substituents compared to each other. The main difference between geminal dihalide and vicinal dihalides is that geminal dihalides have both halide groups attached to the same carbon atom of the compound whereas vicinal dihalides have two halide groups which are attached to two adjacent carbon atoms in the same compound.
Complete step by step solution:
-The word gem dihalide is an abbreviation for geminal dihalide.
-Geminal word in chemistry is derived from a Latin word ‘Gemini’ which means ‘twins’ which refers to the relationship between two atoms or functional groups which are attached to the same atoms.
-The common name for geminal dihalide is alkylidene halides.
-The IUPAC name for the compound in the first option is 2,2 dichloro propane. This compound was shown to produce acute toxicity in rats when subjected to inhalation and have affected sense organs.
-The IUPAC name for the compound in the second option is 1-chloro 2-bromo propane. This compound is sometimes used to determine the enantiomeric resolution of simple chromatographic methods.
-The IUPAC name for the compound in the third option is 1,2-dichloro propane. This compound is also known as propylene dichloride. This is an intermediate formed in the production of perchloroethylene and other chlorinated chemicals. Propylene dichloride was once used as a soil fumigant, chemical intermediate, and as an industrial solvent, but some of these uses have been discontinued. This compound was found to show carcinogenicity. In Japan, there were cases registered in March 2013 for cancer caused by the use of cleaning agents containing 1.2 dichloropropane.
-The IUPAC name for compound in the fourth option is 1,2-dibromo propane which is also known as propylene dibromide which is the simplest carbon hydrocarbon containing two bromine atoms.
So, the correct answer is option (A).
Note: You may get confused between geminal dihalide and vicinal dihalides. The geminal dihalides are organic compounds containing two halide groups attached to the same carbon whereas vicinal dihalides are organic compounds having two halide groups attached to two adjacent carbon atoms of the same chemical compound. The terms ‘geminal’ and ‘vicinal’ are used for chemical compounds having substituents. These terms distinguish chemical compounds based on the location of substituents compared to each other. The main difference between geminal dihalide and vicinal dihalides is that geminal dihalides have both halide groups attached to the same carbon atom of the compound whereas vicinal dihalides have two halide groups which are attached to two adjacent carbon atoms in the same compound.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




