
Which of the following is basic amino acid?
A. Lysine
B. Proline
C. Alanine
D. Aspartic acid
Answer
561k+ views
Hint: To answer this question we should talk about amino acids. The amino acids are made mainly of carbon, hydrogen, nitrogen and oxygen. The amino acids have amine group, carboxylic acid and alkyl chain. In water, the carboxylic acid loses proton and amine gains proton so amino acids behave as acid and base. We will draw the structure of all given amino acids to determine the basic amino acid.
Complete step-by-step answer:
In all amino acids two functional groups are present. One is carboxylic and the second is an amine group. The carboxylic acid donate proton so it behaves as acid and amine has lone pair so it can accept the proton so it behaves as base.
As both acidic part and basic part are present in amino acid so, amino acid behaves as an acid as well as a base in water. It’s carboxylic part donates proton in water simultaneously the basic part gains the proton so, all over the amino acid having one carboxylic group and one amine group behaves as neutral.
In some amino acids the carboxylic acid is one but amine groups are more than one that amino acid donate one proton about gaining one than one proton so they behave as basic.
The structure of the given amino acids are as follows:
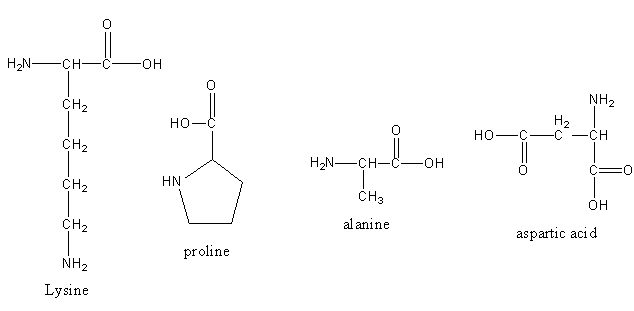
In all the given amino acids, only the lysine is one which has one carboxylic group and two amine groups, so it is the basic amino acid.
Therefore, option (A) Lysine, is correct.
Note: One side of amino acid (having carboxylic acid) gains negative charge after losing proton and another side of amino acid (having amine group) gains positive charge after accepting proton. So, all over charge is zero. This structure of amino acid having oppositely charged sides is known as Zwitterion. Aspartic acid is the acidic amino acid as it has two carboxylic groups and one amine group. Proline and alanine both have one-one carboxylic and amine groups, so both are neutral amino acids.
Complete step-by-step answer:
In all amino acids two functional groups are present. One is carboxylic and the second is an amine group. The carboxylic acid donate proton so it behaves as acid and amine has lone pair so it can accept the proton so it behaves as base.
As both acidic part and basic part are present in amino acid so, amino acid behaves as an acid as well as a base in water. It’s carboxylic part donates proton in water simultaneously the basic part gains the proton so, all over the amino acid having one carboxylic group and one amine group behaves as neutral.
In some amino acids the carboxylic acid is one but amine groups are more than one that amino acid donate one proton about gaining one than one proton so they behave as basic.
The structure of the given amino acids are as follows:

In all the given amino acids, only the lysine is one which has one carboxylic group and two amine groups, so it is the basic amino acid.
Therefore, option (A) Lysine, is correct.
Note: One side of amino acid (having carboxylic acid) gains negative charge after losing proton and another side of amino acid (having amine group) gains positive charge after accepting proton. So, all over charge is zero. This structure of amino acid having oppositely charged sides is known as Zwitterion. Aspartic acid is the acidic amino acid as it has two carboxylic groups and one amine group. Proline and alanine both have one-one carboxylic and amine groups, so both are neutral amino acids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




